Multiwalled carbon nanotubes decorated ɛ-MnO2 nanoflowers cathode with oxygen defect for aqueous zinc-ion batteries
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
As a potential cathode for aqueous zinc-ion batteries, the low electrical conductivity and poor electrochemical kinetics of MnO2 limit its further development and application. Herein, multiwalled carbon nanotubes decorated MnO2 nanoflowers with hexagonal structure (ɛ-MnO2/MWCNTs) were synthesized by one-step co-precipitation method. The ɛ-MnO2/MWCNTs inherit the advantages of highly conductive MWCNTs and nanostructured MnO2, thus showing excellent zinc-ion storage capacity. The ɛ-MnO2/MWCNTs display high invertible capacity of 335.6 mAh/g at 0.2 A/g after 150 cycles, and long-term capacity of 115.7 mAh/g at 2.0 A/g after 4000 cycles. The results of kinetics tests further reveal that the MWCNTs decoration can reduce polarization of MnO2 and accelerate its reaction kinetics. Thanks to these merits, the ɛ-MnO2/MWCNTs hold great potential for high performance eco-friendly batteries cathode material.
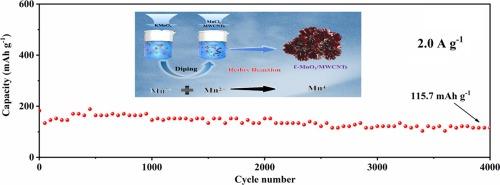
多壁碳纳米管装饰的具有氧缺陷的ɛ-MnO2 纳米流阴极用于锌离子水电池
作为一种潜在的水性锌离子电池阴极,二氧化锰的低导电性和较差的电化学动力学限制了其进一步的开发和应用。本文采用一步共沉淀法合成了具有六方结构的多壁碳纳米管装饰 MnO2 纳米花(ɛ-MnO2/MWCNTs)。ɛ-MnO2/MWCNTs继承了高导电性 MWCNTs 和纳米结构 MnO2 的优点,因此具有优异的锌离子存储能力。经过 150 次循环后,ɛ-MnO2/MWCNTs 在 0.2 A/g 条件下显示出 335.6 mAh/g 的高可逆容量;经过 4000 次循环后,在 2.0 A/g 条件下显示出 115.7 mAh/g 的长期容量。动力学测试结果进一步表明,MWCNTs 装饰可降低 MnO2 的极化,并加快其反应动力学。由于这些优点,ɛ-MnO2/MWCNTs 在高性能环保电池正极材料方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


