Tuning the composition of mixed anthraquinone derivatives towards an affordable flow battery negolyte
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Having a long lifespan and being capable of scaling capacity and power independently, redox flow batteries (RFB) offer great opportunities for energy storage. However, the challenge lies in finding an ideal electrolyte. The most mature version of RFB utilizes vanadium solutions and suffers from rising and highly volatile prices of this metal. To address this, organic electrolytes are gaining attention, as they can be obtained from abundant feedstocks. Among those, Anthraquinone-2,7-disulfonic acid (2,7-AQDS) solutions are particularly prominent, demonstrating reversible and fast redox kinetics coupled with reasonable solubility. This paper explores the possibility of synthesizing 2,7-AQDS together with other electroactive compounds (2,6-AQDS, 2-AQS) through the reaction of anthraquinone sulfonation. It shows that obtained mixtures act as electrolytes without any purification or separation, while synthesis conditions can adjust mixture composition and hence their redox behavior. Although the performance of anthraquinone-bromine RFB utilizing these mixtures exhibits a trade-off between power and stability, the best of them are comparable or even superior to 2,7-AQDS. For instance, RFB with a mixture free of 2-AQS demonstrates an energy efficiency of 76.4 % and a capacity fade rate of 0.04 %/cycle at a current density of 75 mA cm−2. The specific capacity of such mixtures can reach 70 Ah L−1, which makes them promising and affordable RFB negolyte.
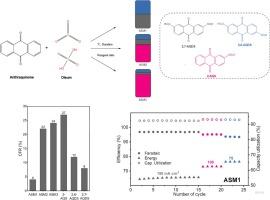
调整混合蒽醌衍生物的成分,开发经济实惠的液流电池负极溶解剂
氧化还原液流电池(RFB)具有使用寿命长、可独立扩展容量和功率等特点,为能源存储提供了巨大的机遇。然而,挑战在于找到理想的电解质。最成熟的氧化还原液流电池使用钒溶液,但这种金属的价格不断上涨且极不稳定。为了解决这个问题,有机电解质受到越来越多的关注,因为它们可以从丰富的原料中获得。其中,蒽醌-2,7-二磺酸(2,7-AQDS)溶液尤为突出,它具有可逆、快速的氧化还原动力学以及合理的溶解度。本文探讨了通过蒽醌磺化反应将 2,7-AQDS 与其他电活性化合物(2,6-AQDS、2-AQS)合成在一起的可能性。研究表明,获得的混合物可作为电解质使用,无需任何纯化或分离,而合成条件可调整混合物的成分,从而调整其氧化还原行为。虽然使用这些混合物的蒽醌溴 RFB 的性能在功率和稳定性之间有所权衡,但其中最好的混合物与 2,7-AQDS 不相上下,甚至更胜一筹。例如,在电流密度为 75 mA cm-2 时,使用不含 2-AQS 的混合物的 RFB 的能量效率为 76.4%,容量衰减率为 0.04%/周期。这种混合物的比容量可达 70 Ah L-1,因此是一种前景广阔、价格合理的 RFB 负极。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


