Boron vs. aluminum in ZSM-5 zeolites: Solid-state NMR, acidity, and C1/C2 reactant conversion
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
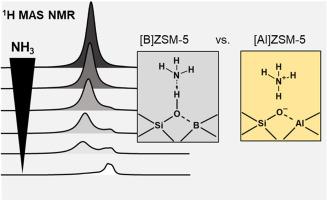
Three zeolite catalysts with comparable amounts of aluminum and/or boron ([Al]ZSM-5, [B,Al]ZSM-5, and [B]ZSM-5) are herein synthesized. Water (H2O), ammonia (NH3), acetonitrile-d3 (ACN), and trimethylphosphine oxide (TMPO) are applied as probe molecules to investigate the acidity of the respective materials in combination with 11B, 27Al, and 29Si MAS NMR spectroscopy. Ammonia is not protonated to ammonium on Si(OH)B groups and only LAS-bound ammonia persists desorption. Thus, ammonia gives a realistic, quantitative picture of the present acid sites. ACN interacts only with Si(OH)Al as desired. The strong base TMPO results in a misleading, not quantifiable picture. Subsequent hydration is unsuited to distinguish BAS and LAS densities. The samples were catalytically tested in the conversion of methanol, ethanol, and ethylene. [B]ZSM-5 is unreactive in hydrocarbon formation due to absence of BAS, instead LAS are present. The mixed [B,Al]ZSM-5 shows a decreased lifetime in MTO conversion compared to the [Al]ZSM-5, due to LAS presence. A sometimes reported superior reactivity of [B,Al]ZSM-5 catalysts is thus explained primarily by an optimized BAS density.
ZSM-5 沸石中的硼与铝:固态核磁共振、酸度和 C1/C2 反应物转换
本文合成了三种铝和/或硼含量相当的沸石催化剂([Al]ZSM-5、[B,Al]ZSM-5 和 [B]ZSM-5)。水 (H2O)、氨 (NH3)、乙腈-d3 (ACN) 和三甲基氧化膦 (TMPO) 被用作探针分子,结合 11B、27Al 和 29Si MAS NMR 光谱来研究各自材料的酸性。氨不会在 Si(OH)B 基团上质子化为铵,只有与 LAS 结合的氨才会持续解吸。因此,氨能真实、定量地反映目前的酸性位点。ACN 只与 Si(OH)Al 起作用。而强碱 TMPO 则会产生误导,无法量化。随后的水合作用无法区分 BAS 和 LAS 密度。这些样品在甲醇、乙醇和乙烯的转化过程中进行了催化测试。由于缺乏 BAS,[B]ZSM-5 在碳氢化合物的形成过程中没有反应,反而存在 LAS。与[Al]ZSM-5 相比,混合[B,Al]ZSM-5 在 MTO 转化过程中的寿命缩短,原因是存在 LAS。因此,[B,Al]ZSM-5 催化剂有时被报道具有更高的反应活性,其主要原因是优化了 BAS 密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


