Tuning the atomic ordering of AFI framework with templates charge
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
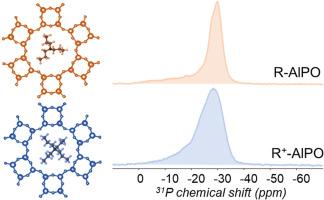
In this work, AlPO4-5 (AFI) zeotype materials were synthesized using the microwave approach in the presence of two organic templates i.e. tripropylamine (R) and tetrapropylammonium (R+). The long- and short-range crystalline order of the samples were investigated by considering the effect of the template's charge on the atomic-level ordering of the AFI framework. Using advanced NMR spectroscopy, we shed the light on the importance of templates' role on the organization of the inorganic framework of aluminophosphates. The spectroscopic data clearly show a wider distribution of Al and P environments in the materials synthesized with the charged template (R+) due to the need of hydroxyl groups charge compensators that induce some bridges distortions. When a charged molecule (R+) was used, aluminum appears to be responsible for the charge compensation mechanism via the association of OH groups, impacting the environment of phosphorus as well. The hydroxyl groups needed for charge compensation in samples synthesized with R+ persist after calcination. Consequently, the hydrophilicity of the sample synthesized with R+ features approximatively two times higher values compared to the one synthesized with R.
用模板电荷调整 AFI 框架的原子排序
本研究采用微波方法,在三丙胺(R)和四丙胺(R+)两种有机模板的存在下合成了 AlPO4-5(AFI)沸石材料。通过考虑模板电荷对 AFI 框架原子级有序的影响,研究了样品的长程和短程晶序。利用先进的核磁共振光谱,我们揭示了模板对铝磷酸盐无机框架组织的重要作用。光谱数据清楚地表明,由于需要羟基电荷补偿器来诱导一些桥梁畸变,在使用带电模板(R+)合成的材料中,Al 和 P 的环境分布更广。当使用带电分子(R+)时,铝似乎通过羟基的结合负责电荷补偿机制,这也影响了磷的环境。在使用 R+ 合成的样品中,电荷补偿所需的羟基在煅烧后仍然存在。因此,与用 R 合成的样品相比,用 R+ 合成的样品的亲水性大约高出两倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


