Hydroxylation of an ultrathin Co3O4(111) film on Ir(100) studied by in situ ambient pressure XPS and DFT
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In the present work, we have studied the interaction of water with spinel cobalt oxide (Co3O4), an effect which has been considered a major cause of its catalytic deactivation. Employing a Co3O4(111) model thin film grown on Ir(100) in (ultra)high vacuum, and ambient pressure X-ray photoelectron spectroscopy (APXPS), hydroxylation in 0.5 mbar H2O vapor at room temperature was monitored in real time. The surface hydroxyl (OH) coverage was determined via two different models based (i) on the termination of a pristine and OH-covered Co3O4(111) surface as derived from density functional theory (DFT) calculations, and (ii) on a homogeneous cobalt oxyhydroxide (CoO(OH)) overlayer. Langmuir pseudo-second-order kinetics were applied to characterize the OH evolution with time, suggesting two regimes of chemisorption at the mosaic-like Co3O4(111) film: (i) plateaus, which were quickly saturated by OH, followed by (ii) slow hydroxylation in the “cracks” of the thin film. H2O dissociation and OH formation, blocking exposed Co2+ ions and additionally consuming surface lattice oxygen, respectively, may thus account for catalyst deactivation by H2O traces in reactive feeds.
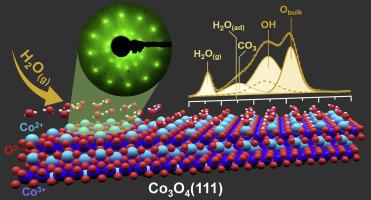
通过原位常压 XPS 和 DFT 研究 Ir(100) 上超薄 Co3O4(111) 薄膜的羟基化过程
在本研究中,我们研究了水与尖晶石氧化钴(Co3O4)的相互作用,这种作用一直被认为是导致其催化失活的主要原因。利用在(超)高真空条件下在 Ir(100)上生长的 Co3O4(111)模型薄膜和环境压力 X 射线光电子能谱(APXPS),对室温下 0.5 毫巴 H2O 蒸汽中的羟基化进行了实时监测。通过两种不同的模型确定了表面羟基(OH)的覆盖率,这两种模型分别基于(i)密度泛函理论(DFT)计算得出的原始表面和羟基覆盖的 Co3O4(111)表面的终止,以及(ii)均匀的氧氢氧化钴(CoO(OH))覆盖层。兰缪尔假秒阶动力学用于描述 OH 随时间的演化过程,表明在镶嵌状 Co3O4(111) 薄膜上存在两种化学吸附状态:(i) 高原,OH 迅速饱和;(ii) 薄膜 "裂缝 "中的缓慢羟基化。因此,H2O 解离和 OH 形成分别阻挡了暴露在外的 Co2+ 离子,并额外消耗了表面晶格氧,这可能是反应性进料中 H2O 痕迹导致催化剂失活的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


