Biocompatible, controlled-release remdesivir-loaded liposomes tackling the telomerase activity of Non-Small cell lung cancer cells: Preparation, characterization, in vitro biological evaluation, and molecular docking analysis
IF 3.6
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
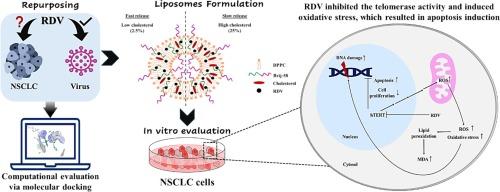
Non-small cell lung cancer (NSCLC) is a global leading cause of cancer mortality. Herein, remdesivir (RDV) was loaded into biocompatible liposomes (RDV-Lips) composed of 1,2-dipalmitoyl-sn‑glycero-3-phosphocholine (DPPC), cholesterol, and polyethylene glycol hexadecyl ether (Brij-58) to enhance its solubility and anticancer efficiency. The study highlighted the possible RDV-induced underlying events, emphasizing its inhibitory potential of telomerase activity through in-silico docking and in vitro studies. RDV-Lips were developed using thin-film hydration and then subjected to physicochemical characterizations. The selected formulations were evaluated for their stability, in vitro release, and in vitro anticancer activity. The size range of RDV-Lips was 83.8–157.9 nm with a polydispersity index (PDI) lower than 0.23 and entrapment exceeded 93%. The cholesterol content of RDV-Lips offered a control point of RDV release, where high and low concentrations exerted slow and fast release patterns, respectively. RDV-Lips showed enhanced anticancer activity and selectivity. They inhibited colony formation, increased lipid peroxidation, induced apoptosis, and inhibited the telomerase activity in a dose-dependent manner. In conclusion, RDV-Lips overcame RDV solubility problems and enhanced its anticancer efficiency. RDV could be a potential therapy against NSCLC via induction of oxidative stress and inhibition of the telomerase activity, which, in turn, restricts unlimited cellular proliferation and apoptosis induction.
具有生物相容性的控释雷米替韦脂质体可抑制非小细胞肺癌细胞的端粒酶活性:制备、表征、体外生物学评价和分子对接分析
非小细胞肺癌(NSCLC)是全球癌症死亡的主要原因。在本研究中,雷米替韦(RDV)被载入由1,2-二棕榈酰-sn-甘油-3-磷酸胆碱(DPPC)、胆固醇和聚乙二醇十六烷基醚(Brij-58)组成的生物相容性脂质体(RDV-Lips)中,以提高其溶解度和抗癌效率。该研究强调了 RDV 可能诱导的基本事件,并通过室内对接和体外研究强调了其抑制端粒酶活性的潜力。研究人员利用薄膜水合技术开发了 RDV-Lips,并对其进行了理化表征。对所选制剂的稳定性、体外释放和体外抗癌活性进行了评估。RDV-Lips 的尺寸范围为 83.8-157.9 nm,多分散指数(PDI)低于 0.23,夹带率超过 93%。RDV-Lips 的胆固醇含量提供了一个 RDV 释放的控制点,高浓度和低浓度分别具有缓慢和快速的释放模式。RDV-Lips 显示出更强的抗癌活性和选择性。它们以剂量依赖的方式抑制了癌细胞的形成,增加了脂质过氧化,诱导了细胞凋亡,并抑制了端粒酶的活性。总之,RDV-Lips 克服了 RDV 的溶解性问题,提高了其抗癌效率。RDV 可以通过诱导氧化应激和抑制端粒酶活性,进而限制细胞无限增殖和诱导细胞凋亡,成为一种潜在的治疗 NSCLC 的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Biotechnology
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
6.70
自引率
3.60%
发文量
50
审稿时长
38 days
期刊介绍:
Current Research in Biotechnology (CRBIOT) is a new primary research, gold open access journal from Elsevier. CRBIOT publishes original papers, reviews, and short communications (including viewpoints and perspectives) resulting from research in biotechnology and biotech-associated disciplines.
Current Research in Biotechnology is a peer-reviewed gold open access (OA) journal and upon acceptance all articles are permanently and freely available. It is a companion to the highly regarded review journal Current Opinion in Biotechnology (2018 CiteScore 8.450) and is part of the Current Opinion and Research (CO+RE) suite of journals. All CO+RE journals leverage the Current Opinion legacy-of editorial excellence, high-impact, and global reach-to ensure they are a widely read resource that is integral to scientists' workflow.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


