Autoencoder-based inverse design and surrogate-based optimization of an integrated wet granulation manufacturing process
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In pharmaceutical manufacturing, integrating model-based design and optimization can be beneficial for accelerating process development. This study explores the utilization of Machine Learning (ML) techniques as a surrogate model for the optimization of a three-unit wet-granulation based flowsheet model for solid dosage form manufacturing. First, a reduced representation of a wet granulation flowsheet model is developed, incorporating a granulation and milling process, along with a novel dissolution model that accounts for the effect of particle size, porosity, and microstructure on dissolution rate. Two optimization approaches are compared, including an autoencoder-based inverse design and a surrogate-based forward optimization. Both methods address the bi-objective problem of maximizing dissolution time and product yield by identifying the optimal granulation and mill process parameters. For this case study, both approaches were effective and incurred a similar computational cost, averaging under 4 s. However, the autoencoder approach offers an advantage through dimensionality reduction, a feature not available in surrogate-based optimization. Dimensional reduction is particularly beneficial for complex process designs with numerous inputs and outputs. The lower dimensional representation helps improve process understanding through enhanced visualization of the process design space and facilitates feasibility studies involving multiple constraints. The autoencoder-based inverse design introduced in this work showcases an implementation of AI and ML in pharmaceutical process development, demonstrating the potential to enhance process efficiency and product quality in complex manufacturing scenarios.
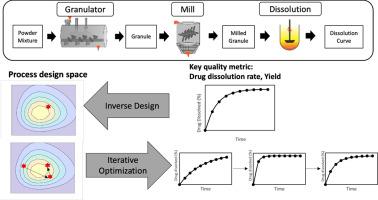
基于自动编码器的湿法造粒集成制造工艺的逆向设计和代用优化
在制药过程中,将基于模型的设计与优化结合起来有利于加速工艺开发。本研究探讨了如何利用机器学习(ML)技术作为代用模型,优化固体制剂生产中基于湿法制粒的三单元流程表模型。首先,开发了湿制粒流程表模型的简化表示法,其中包含制粒和研磨过程,以及考虑到粒度、孔隙率和微观结构对溶出率影响的新型溶出模型。比较了两种优化方法,包括基于自动编码器的逆向设计和基于代理的正向优化。这两种方法都通过确定最佳制粒和研磨工艺参数来解决溶解时间和产品产量最大化的双目标问题。然而,自动编码器方法通过降维提供了优势,这是基于代理的优化所不具备的功能。降维对于具有大量输入和输出的复杂流程设计尤为有利。低维表示法有助于通过增强工艺设计空间的可视化来提高对工艺的理解,并促进涉及多个约束条件的可行性研究。这项工作中介绍的基于自动编码器的逆向设计展示了人工智能和 ML 在制药工艺开发中的应用,证明了在复杂的生产场景中提高工艺效率和产品质量的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


