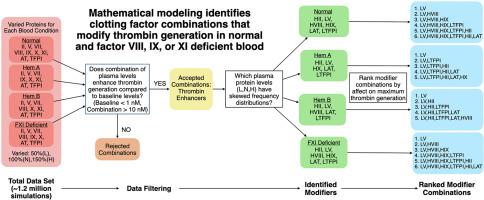
Mathematical modeling identifies clotting factor combinations that modify thrombin generation in normal and factor VIII-, IX-, or XI-deficient blood
IF 3.4
3区 医学
Q2 HEMATOLOGY
Research and Practice in Thrombosis and Haemostasis
Pub Date : 2024-10-01
DOI:10.1016/j.rpth.2024.102570
引用次数: 0
Abstract
Background
In healthy individuals, plasma levels of clotting proteins naturally vary within a range of 50% to 150% of their mean values. We do not know how these variations modify thrombin generation.
Objectives
To assess the impact of protein level variations on simulated thrombin generation in normal and factor (F)VIII-, FIX-, or FXI-deficient blood.
Methods
We used a mathematical model of flow-mediated coagulation to simulate thrombin generation with all possible combinations of clotting protein variations within the normal range and for various tissue factor levels. We selected, analyzed, and ranked combinations that enhanced thrombin generation compared with baseline.
Results
Protein variations most strongly affected thrombin generation at intermediate tissue factor levels. Low tissue factor levels prevented coagulation initiation, while high tissue factor levels always triggered thrombin generation. At intermediate levels, we identified protein variations that substantially modified thrombin generation. Low-normal FV shortened lag times and increased thrombin generation, whereas high-normal FV lengthened lag times and reduced thrombin generation. With severe FVIII and FIX deficiencies, low-normal tissue factor pathway inhibitor α and antithrombin amplified the effect of low-normal FV. For moderate FVIII and FIX deficiencies, high-normal tissue factor pathway inhibitor α and antithrombin enhanced the impact of high-normal FV in reducing thrombin production. In normal and FXI-deficient blood, high-normal FVIII and FIX significantly boosted thrombin generation.
Conclusion
Our mathematical model predicted how variations in clotting protein levels, within the normal range, could contribute to the variability of bleeding phenotypes observed with clotting factor deficiencies. Our study generated experimentally testable hypotheses that could aid in developing new therapies toward normal hemostasis.
数学建模确定可改变正常血液和因子 VIII、IX 或 XI 缺乏血液中凝血酶生成的凝血因子组合
背景在健康人体内,凝血蛋白的血浆水平自然会在其平均值的50%至150%范围内变化。我们使用流动介导凝血的数学模型来模拟凝血酶的生成,其中包括正常范围内凝血蛋白变化的所有可能组合以及各种组织因子水平的变化。我们选择、分析了与基线相比能增强凝血酶生成的组合,并对其进行了排序。结果在中等组织因子水平时,蛋白变化对凝血酶生成的影响最大。低组织因子水平阻止了凝血的开始,而高组织因子水平则总是触发凝血酶的生成。在中等水平时,我们发现蛋白质变异会显著改变凝血酶的生成。低正常 FV 缩短了滞后时间并增加了凝血酶的生成,而高正常 FV 则延长了滞后时间并减少了凝血酶的生成。在 FVIII 和 FIX 严重缺乏的情况下,低正常值的组织因子通路抑制剂 α 和抗凝血酶会放大低正常值 FV 的效应。对于中度 FVIII 和 FIX 缺乏症,高正常组织因子途径抑制剂 α 和抗凝血酶增强了高正常 FV 在减少凝血酶生成方面的作用。结论我们的数学模型预测了凝血蛋白水平在正常范围内的变化如何导致凝血因子缺乏症出血表型的变化。我们的研究提出了可通过实验验证的假设,这些假设有助于开发新的疗法,以实现正常止血。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Research and Practice in Thrombosis and Haemostasis
Medicine-Hematology
CiteScore
5.60
自引率
13.00%
发文量
212
审稿时长
7 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


