FTIR, 1H-/13C-NMR spectral characterization, antimicrobial, anticancer, antioxidant, anti-inflammatory, PASS, SAR, and in silico properties of methyl α-D-glucopyranoside derivatives
IF 3.8
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
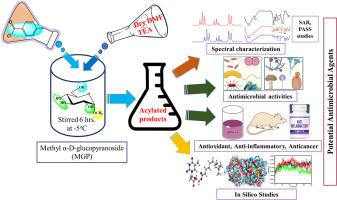
A novel series of biologically active derivatives based on methyl α-D-glucopyranoside (MGP) has been developed, comprising 6-O-monosubstituted MGP derivatives obtained from methyl α-D-glucopyranoside. These derivatives were transformed into 2,3,4-tri-O-acyl MGP derivatives, incorporating diverse functionalities within a single molecular framework, aimed at producing new products for antimicrobial studies. All synthesized compounds were identified through spectral analyses (FTIR, 1H-NMR, and 13C-NMR) and elemental analysis. Antimicrobial in vitro testing revealed that these MGP derivatives have notable efficacy against various pathogenic microorganisms, along with the prediction of activity spectra for substances (PASS). Compounds 2 and 7 exhibited the highest inhibitory activity against Bacillus subtilis and Escherichia coli, with minimum inhibitory concentration (MIC) values ranging from 0.25 to 64.0 µg/mL and minimum bactericidal concentration (MBC) values ranging from 8.0 to 128.0 µg/mL. Moreover, these compounds demonstrated significant antioxidant properties compared with those of standard antioxidants according to the results of the DPPH free radical scavenging assay. An evaluation of the growth and proliferation of Ehrlich ascites carcinoma (EAC) cells revealed moderate cell growth inhibition by compounds 5 and 6, with IC50 values of 5958.54 and 5437.17 µg/mL, respectively, as determined via an MTT colorimetric assay. An analysis of the structure-activity relationship (SAR) revealed that the combination of the (p-CH3.C6H4CO-) and halo-aromatic [3-Cl.C6H4CO-] chains with sugar had the highest efficiency in pathogens. Molecular docking studies using AutoDock Vina highlighted compound 7 as a promising inhibitor of the carbapenemase, OmpF, and HmoB proteins, with binding energies of -11.53 kcal/mol, -2.26 kcal/mol, and -30.75 kcal/mol, respectively. A 100-ns molecular dynamics simulation study demonstrated the validity of stable conformation and binding patterns in a stimulating environment. Pharmacokinetic characterization and ADMET predictions indicated favorable drug-like properties. These substantial in vitro and in silico studies demonstrate the importance of additional investigations to confirm the efficacy of MGP derivatives as antimicrobial agents.
甲基α-D-吡喃葡萄糖苷衍生物的傅里叶变换红外光谱、1H-/13C-NMR 光谱特性、抗菌、抗癌、抗氧化、抗炎、PASS、SAR 和硅学特性
以甲基α-D-吡喃葡萄糖苷(MGP)为基础,开发了一系列具有生物活性的新型衍生物,其中包括从甲基α-D-吡喃葡萄糖苷中获得的 6-O-单取代 MGP 衍生物。这些衍生物被转化为 2,3,4-三-O-酰基 MGP 衍生物,在单个分子框架内整合了多种功能,旨在生产出用于抗菌研究的新产品。通过光谱分析(傅立叶变换红外光谱、1H-NMR 和 13C-NMR)和元素分析,确定了所有合成化合物。抗菌体外测试表明,这些 MGP 衍生物对各种病原微生物具有显著疗效,同时还对物质的活性光谱进行了预测(PASS)。化合物 2 和 7 对枯草杆菌和大肠杆菌的抑制活性最高,最低抑菌浓度 (MIC) 值为 0.25 至 64.0 µg/mL,最低杀菌浓度 (MBC) 值为 8.0 至 128.0 µg/mL。此外,根据 DPPH 自由基清除试验的结果,与标准抗氧化剂相比,这些化合物具有显著的抗氧化特性。通过 MTT 比色法对艾氏腹水癌(EAC)细胞的生长和增殖进行评估后发现,化合物 5 和 6 对细胞生长有适度的抑制作用,IC50 值分别为 5958.54 和 5437.17 µg/mL。结构-活性关系(SAR)分析表明,(p-CH3.C6H4CO-)和卤代芳香族[3-Cl.C6H4CO-]链与糖的组合对病原体的作用效率最高。使用 AutoDock Vina 进行的分子对接研究表明,化合物 7 是一种很有希望的碳青霉烯酶、OmpF 和 HmoB 蛋白抑制剂,其结合能分别为 -11.53 kcal/mol、-2.26 kcal/mol 和 -30.75 kcal/mol。一项 100-ns 的分子动力学模拟研究证明了在刺激环境中稳定构象和结合模式的有效性。药代动力学表征和 ADMET 预测表明,该药物具有良好的类药物特性。这些大量的体外和硅学研究表明,必须进行更多的研究来确认 MGP 衍生物作为抗菌剂的功效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


