Synthesis of hydrophobic polymeric surfactant (Polyacrylamide/Zwitterionic) and its effect on enhanced oil recovery (EOR)
IF 3.8
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
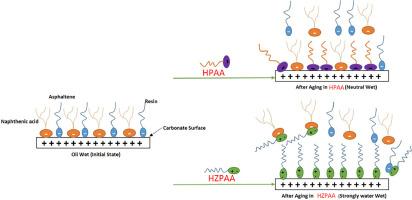
Injection of surfactant and polymer at the same time is one of the challenges that reduce their performance in reservoirs. Using a polymeric surfactant (PS) as a single new material can be the best alternative to solve the existing challenges. Considering the basic problems of chemical injection, this work focuses on the surface adsorption of polymer/surfactant on carbonate reservoirs (CRs). In this work, polyacrylamide was synthesized using zwitterionic as a hydrophobic polymeric surfactant (i.e. HZPAA). For a better comparison, this substance was compared with hydrolyzed polyacrylamide (HPAA) in concentrations of 40 to 1000 mg in CRs. Examining the results states that with increasing polymer concentration, the surface absorption of HZPAA and HPAA on dolomite reservoir rocks (DRRs) with positive surface charge increases. The attendance of COO and SO3− functional groups in the matrix of the new polymer (i.e. HZPAA) creates electrostatic forces and superior surface absorption. Their mechanism works in such a way that negative functional groups attract positively charged surfaces and increase the surface absorption of the polymer. Investigations show that the new synthesized material (i.e. HZPAA) can provide a new approach to improving surface absorption in carbonate reservoirs.
疏水性聚合物表面活性剂(聚丙烯酰胺/齐聚物)的合成及其对提高石油采收率(EOR)的影响
同时注入表面活性剂和聚合物是降低其在油藏中性能的难题之一。使用聚合物表面活性剂(PS)作为单一的新材料是解决现有难题的最佳选择。考虑到化学注水的基本问题,这项工作重点关注聚合物/表面活性剂在碳酸盐岩储层(CRs)上的表面吸附。在这项工作中,聚丙烯酰胺的合成使用了作为疏水性聚合物表面活性剂的齐聚物(即 HZPAA)。为了更好地进行比较,将这种物质与 CR 中浓度为 40 至 1000 毫克的水解聚丙烯酰胺(HPAA)进行了比较。研究结果表明,随着聚合物浓度的增加,HZPAA 和 HPAA 在表面带正电荷的白云岩储层岩石(DRR)上的表面吸收率也会增加。新聚合物(即 HZPAA)基质中 COO 和 SO3- 官能团的加入产生了静电力和优异的表面吸收能力。它们的作用机理是负功能基团吸引带正电的表面,增加聚合物的表面吸收能力。研究表明,新合成的材料(即 HZPAA)可以为改善碳酸盐储层的表面吸收提供一种新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


