Study of Two-Photon induced excited state absorption of Three-Branched triphenylamine Derivatives: Cooperative and Anti-Cooperative effect of electron transition in the excited state
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-06
DOI:10.1016/j.jphotochem.2024.116078
引用次数: 0
Abstract
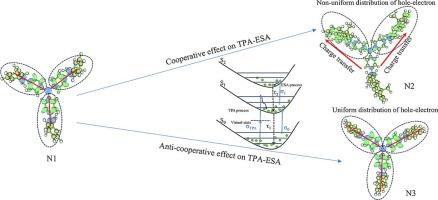
For multi-branched molecules, intramolecular cooperative effect can significantly enhance the molecular nonlinear optical absorption. Three triphenylamine-cored compounds (N1, N2 and N3) with three branches are synthesized to study the cooperative and anti-cooperative effect of electron transition in the excited state on two-photon absorption (TPA) and excited state absorption (ESA). Molecular polarization of these multi-branched triphenylamine derivatives is regulated by changing the molecular symmetry and the planarity of peripheral branches, to regulate their charge distribution and electron transition characteristics in the excited state. Here, we show that due to electronic coupling and interaction between certain branches, the asymmetric distribution of electron clouds in the excited states of these multi-branched molecules will lead to an enhancement of their TPA and ESA cross-sections, which is known as the cooperative effect of electron transitions. On the contrary, electronic coupling and interaction among all branches will lead to a highly symmetric distribution of electron clouds in the excited states of these multi-branched molecules, which will cause anti-cooperative effects and result in significant attenuation of TPA and ESA cross-sections.
三苯胺衍生物的双光子诱导激发态吸收研究:激发态电子转变的合作与反合作效应
对于多分支分子,分子内的协同效应可以显著增强分子的非线性光学吸收。本文合成了三种具有三个分支的三苯胺涂层化合物(N1、N2 和 N3),以研究激发态电子跃迁对双光子吸收(TPA)和激发态吸收(ESA)的协同和反协同效应。通过改变分子对称性和外围分支的平面度来调节这些多分支三苯胺衍生物的分子极化,从而调节它们在激发态的电荷分布和电子跃迁特性。在这里,我们展示了由于某些分支之间的电子耦合和相互作用,这些多分支分子激发态电子云的不对称分布将导致其 TPA 和 ESA 截面的增强,这就是所谓的电子跃迁的合作效应。相反,所有分支之间的电子耦合和相互作用将导致这些多分支分子激发态中电子云的高度对称分布,从而产生反合作效应,使 TPA 和 ESA 截面显著衰减。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


