Efficient photocatalytic degradation of chloramphenicol from contaminated water over rGO@MoS2 nanocomposites
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-09
DOI:10.1016/j.jphotochem.2024.116076
引用次数: 0
Abstract
Antibiotics-based effluents pose a severe threat to the natural ecosystem by acting as reservoirs for the growth of drug-resistant bacteria if untreated. Herein, a facile and scalable approach was developed to engineer photocatalysts associating reduced graphene oxide (rGO) and molybdenum disulfide (rGO@MoS2) that were used for the degradation of chloramphenicol (CAP) from contaminated water under UV-light. The hydrothermally produced rGO@MoS2 catalysts contain C![]() O, C
O, C![]() OH, and C
OH, and C![]() O
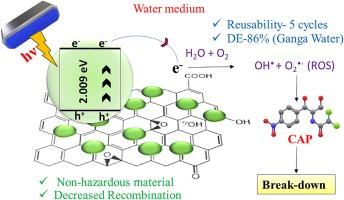
O![]() C functional groups that contribute to the binding of CAP onto their surface. The rGO surface modification with MoS2 layers offers a high surface area for light absorption. Results show that the rGO@MoS2 catalyst (1:1 w/w) exhibits the highest degradation efficiency (DE) of 86 % within 180 min of light exposure at neutral pH. Further, the kinetic modelling studies for CAP degradation by rGO@MoS2 1:1 showed high linearity with pseudo-first order kinetics (R2 ∼ 0.9). The isotherm modelling studies correspond to Langmuir isotherm (R2 ∼ 0.99), suggesting monolayer-type interaction of CAP with the photocatalyst surface during photodegradation. Furthermore, the mechanism of degradation elucidated using scavenging experiments showed the involvement of both holes (h+) and electrons (e−) that contribute to the degradation of CAP by generating reactive oxygen species (ROS) like OH● and O2●− radicals. The applicability of the rGO@MoS2 photocatalyst towards the degradation of CAP was tested in Ganga water, wherein a similar ∼86 % CAP removal was observed. Furthermore, the photocatalyst was found to be stable and could be reused five times. Overall, these findings demonstrate the usefulness of the rGO@MoS2 1:1 composite as an efficient photocatalyst that can be used for the degradation of harmful organic pollutants from water bodies to provide a safer and cleaner environment.
C functional groups that contribute to the binding of CAP onto their surface. The rGO surface modification with MoS2 layers offers a high surface area for light absorption. Results show that the rGO@MoS2 catalyst (1:1 w/w) exhibits the highest degradation efficiency (DE) of 86 % within 180 min of light exposure at neutral pH. Further, the kinetic modelling studies for CAP degradation by rGO@MoS2 1:1 showed high linearity with pseudo-first order kinetics (R2 ∼ 0.9). The isotherm modelling studies correspond to Langmuir isotherm (R2 ∼ 0.99), suggesting monolayer-type interaction of CAP with the photocatalyst surface during photodegradation. Furthermore, the mechanism of degradation elucidated using scavenging experiments showed the involvement of both holes (h+) and electrons (e−) that contribute to the degradation of CAP by generating reactive oxygen species (ROS) like OH● and O2●− radicals. The applicability of the rGO@MoS2 photocatalyst towards the degradation of CAP was tested in Ganga water, wherein a similar ∼86 % CAP removal was observed. Furthermore, the photocatalyst was found to be stable and could be reused five times. Overall, these findings demonstrate the usefulness of the rGO@MoS2 1:1 composite as an efficient photocatalyst that can be used for the degradation of harmful organic pollutants from water bodies to provide a safer and cleaner environment.
利用 rGO@MoS2 纳米复合材料高效光催化降解污染水中的氯霉素
以抗生素为基础的污水对自然生态系统构成了严重威胁,因为如果不加以处理,这些污水就会成为耐药细菌滋生的温床。在此,研究人员开发了一种简便、可扩展的方法,将还原氧化石墨烯(rGO)和二硫化钼(rGO@MoS2)结合在一起,制成光催化剂,用于在紫外光下降解污染水中的氯霉素(CAP)。水热法制备的 rGO@MoS2 催化剂含有 CO、COH 和 COC 官能团,有助于将 CAP 结合到其表面。用 MoS2 层对 rGO 表面进行修饰可提供高表面积,有利于光吸收。结果表明,rGO@MoS2 催化剂(1:1 w/w)的降解效率(DE)最高,在中性 pH 值下,光照射 180 分钟内降解效率达到 86%。此外,对 rGO@MoS2 1:1 降解 CAP 的动力学建模研究表明,该催化剂与假一阶动力学具有高度线性关系(R2 ∼ 0.9)。等温线模型研究与 Langmuir 等温线(R2 ∼ 0.99)一致,表明光降解过程中 CAP 与光催化剂表面的相互作用为单层型。此外,利用清除实验阐明的降解机理表明,空穴(h+)和电子(e-)都参与了 CAP 的降解,它们通过产生活性氧(ROS),如 OH● 和 O2●- 自由基,促进了 CAP 的降解。在恒河水中测试了 rGO@MoS2 光催化剂降解 CAP 的适用性,观察到类似的 CAP 去除率为 ∼86%。此外,还发现这种光催化剂非常稳定,可以重复使用五次。总之,这些研究结果证明了 rGO@MoS2 1:1 复合材料作为一种高效光催化剂的实用性,可用于降解水体中的有害有机污染物,从而提供更安全、更清洁的环境。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


