A cradle-to-gate life cycle assessment for clean hydrogen gas production pathway using the CeO2/Ce2O3-based redox thermochemical cycle
0 ENERGY & FUELS
引用次数: 0
Abstract
The present study is conducted to develop a two-steps redox thermochemical cycle based on combined ceria reduction and methane reforming through the CeO2/Ce2O3 redox pair. This cycle incorporates simultaneous water splitting for hydrogen production. To enhance the accuracy and reliability of the inventory data, the system is simulated using the Aspen Plus software package. Also, the goal of this work is to evaluate the carbon footprint of thermochemical based hydrogen production under different scenarios and to compare it with traditional technologies like steam methane reforming (SMR). The analysis takes into account both the plant manufacturing line (reactors), fuel processing (H2 production), redox particles oxygen carrier manufacturing (OC), H2 compression (power consumption) and H2 storage (pipeline manufacturing). The openLCA software with the European database environmental footprint is employed to compute the total carbon emissions of this thermochemical cycle during its overall life cycle. The study concludes that the base case scenario of this system has an overall GWP of 1751.14 g CO2 eq./kg H2 respectively. In the overall GWP, the share of plant construction and OC production was found to be around 1.67%, and 15.78% respectively. The conventional steam methane reforming is loaded with an overall GWP of 4472.83 g CO2 eq./kg H2. In terms of renewable energy input, the base case scenario of this hydrogen production system results in 1465.73 g CO2 eq./kg H2 with thermal energy produced from renewable sources. Comparative life cycle assessment (LCA) demonstrates that base case of CeO2/Ce2O3 has an 85.41% lower GWP emphasizing its environmental advantages over the SMR and Fe3O4/FeO redox pair carries an 84.06% lower GWP as compared to the conventional SMR with 1483.55 g CO2 eq./kg H2. The present study concludes that the combined ceria reduction and methane reforming chemical looping process with simultaneous water splitting may be a more promising option to produce hydrogen using renewable energy.
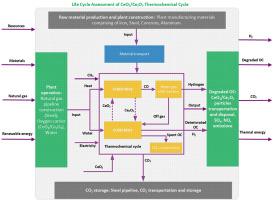
利用基于 CeO2/Ce2O3 的氧化还原热化学循环对清洁氢气生产途径进行从摇篮到终点的生命周期评估
本研究旨在通过 CeO2/Ce2O3 氧化还原对,开发一种基于铈还原和甲烷重整的两步氧化还原热化学循环。该循环结合了同步水分裂制氢。为了提高库存数据的准确性和可靠性,我们使用 Aspen Plus 软件包对该系统进行了模拟。此外,这项工作的目标是评估在不同情况下热化学制氢的碳足迹,并将其与蒸汽甲烷重整(SMR)等传统技术进行比较。分析同时考虑了工厂生产线(反应器)、燃料处理(氢气生产)、氧化还原粒子氧载体制造(OC)、氢气压缩(电力消耗)和氢气储存(管道制造)。研究采用了带有欧洲数据库环境足迹的 openLCA 软件,以计算该热化学循环在其整个生命周期内的碳排放总量。研究得出的结论是,该系统的基础方案的总 GWP 分别为 1751.14 g CO2 eq./kg H2。在总的全球升温潜能值中,工厂建设和 OC 生产所占的比例分别约为 1.67% 和 15.78%。传统蒸汽甲烷转化的总全球升温潜能值为 4472.83 克 CO2 当量/千克 H2。在可再生能源输入方面,该制氢系统的基础方案是利用可再生能源生产热能,结果为 1465.73 g CO2 eq./kg H2。生命周期比较评估(LCA)表明,CeO2/Ce2O3 基准方案的全球升温潜能值比 SMR 低 85.41%,突出了其环境优势;与传统 SMR 相比,Fe3O4/FeO 氧化还原对的全球升温潜能值低 84.06%,为 1483.55 克 CO2 当量/千克 H2。本研究的结论是,结合铈还原和甲烷重整化学循环过程,同时进行水分裂,可能是利用可再生能源制氢的更有前途的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


