Development of Chitosan-Maize Starch based Co-processed excipient: As release retardant material
IF 6.2
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2024-10-02
DOI:10.1016/j.carpta.2024.100580
引用次数: 0
Abstract
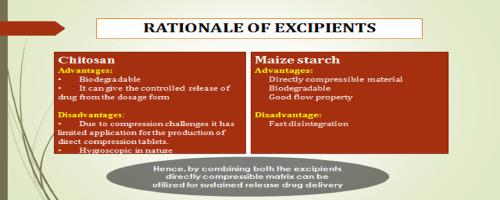
In the present study, co-processed excipients from chitosan, and maize starch were prepared by co-dispersion, and co-granulation methods in 1:1 ratio. The resultant products were then characterized physically by their micrometric properties and hygroscopic characters. The co-processed excipient prepared by co-granulation was chosen and was added to a directly compressible oral sustained-release tablet (Batch F1-F4) containing ibuprofen as a model drug. A well-known marketed ibuprofen tablet (Motrin) was taken for comparison purposes of the dissolution characteristics. Pre-compression parameters, as well as post-compression parameters of all the batches, were performed. The pre-compression parameters, and post-compression parameters of F3, containing chitosan to maize in the ratio of 2:1, were found to be comparable with the marketed product. F3 batch showed 74.7 % of drug release up to 8 h, which reflect the release retardant properties of co-processed excipient. Collectively, co-processed excipients of chitosan, and maize starch improve the physical properties of individual materials, and can be used as a directly compressible excipient as a release retardant material for oral sustained-release tablets.
开发壳聚糖-玉米淀粉共处理辅料:作为缓释材料
本研究采用共分散和共制粒方法,以 1:1 的比例制备了壳聚糖和玉米淀粉共处理辅料。然后通过微量特性和吸湿特性对所得产品进行物理表征。选择共制粒法制备的共加工辅料,并将其添加到含有布洛芬作为模型药物的直接可压缩口服缓释片(批次 F1-F4)中。为了比较溶出特性,采用了一种知名的市场上销售的布洛芬片剂(Motrin)。对所有批次的压缩前参数和压缩后参数进行了测定。结果发现,含有壳聚糖和玉米(比例为 2:1)的 F3 批次的压缩前参数和压缩后参数与市售产品相当。F3 批次在 8 小时内的药物释放率为 74.7%,这反映了共处理辅料的缓释特性。总之,壳聚糖和玉米淀粉共加工辅料改善了单个材料的物理性质,可作为直接可压缩辅料作为缓释材料用于口服缓释片。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


