KRT14 as a potential prognostic marker for metastasis in metaplastic breast carcinoma
IF 0.5
Q4 GENETICS & HEREDITY
引用次数: 0
Abstract
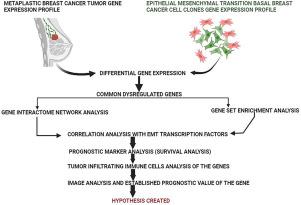
Metaplastic breast cancer (MBC) is an aggressive breast cancer subtype with poor prognosis. To elucidate underlying genomic aberrations driving MBC, we conducted integrated transcriptomic analyses of MBC tumors and basal breast cancer cell lines with various transitioning phases (xE = epithelial phase clone, xM = mesenchymal phase clone and xEM = Epithelial-mesenchymal clone). Differential gene expression analysis between MBC vs non-MBC samples as well as between epithelial-mesenchymal (xEM) transitioning cells vs parental cells revealed 12 commonly dysregulated genes intersected from both the datasets meditates pathways governing EMT, migration, proliferation and metastasis. Further investigation onto those genes based on multi-omics approaches that include survival analysis, correlated expression patterns and interactomics indicated KRT14 had a key role in promoting MBC aggression through Epithelial mesenchymal transition leading to metastasis. Moreover, NPR3 was found to be a possible link between KRT14 and EMT in metastasis. To our knowledge, this is the first evident gene expression study to investigate the potential association between the expression of KRT14 and NPR3 and metastasis in MBC, and their potential involvement in the epithelial-mesenchymal transition (EMT) process. These mechanistic and prognostic insights gained can lead to targeted therapeutics against this rare, refractory disease. Overall, this work highlights the effectiveness of integrated multi-omics in revealing gene expression pattern and predictive biomarkers for metaplastic breast cancer subtypes with poor prognosis.
KRT14 作为移行细胞乳腺癌转移的潜在预后标志物
移行细胞乳腺癌(MBC)是一种侵袭性乳腺癌亚型,预后较差。为了阐明驱动 MBC 的潜在基因组畸变,我们对 MBC 肿瘤和具有不同转化阶段的基础乳腺癌细胞系(xE = 上皮期克隆、xM = 间充质期克隆和 xEM = 上皮-间充质克隆)进行了综合转录组分析。MBC 与非 MBC 样本之间以及上皮-间质(xEM)转化细胞与亲代细胞之间的差异基因表达分析表明,这两个数据集中的 12 个常见失调基因与 EMT、迁移、增殖和转移通路有关。基于多组学方法(包括生存分析、相关表达模式和相互作用组学)对这些基因的进一步研究表明,KRT14 在通过上皮间质转化促进 MBC 侵袭并导致转移方面起着关键作用。此外,研究还发现 NPR3 可能是 KRT14 与 EMT 转移之间的联系。据我们所知,这是首次对 KRT14 和 NPR3 的表达与 MBC 转移之间的潜在联系以及它们在上皮-间质转化(EMT)过程中的潜在参与进行的明显基因表达研究。所获得的这些机理和预后方面的见解将有助于开发针对这种罕见难治性疾病的靶向疗法。总之,这项工作突出了综合多组学在揭示预后不良的移行细胞乳腺癌亚型的基因表达模式和预测性生物标志物方面的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Human Gene
Biochemistry, Genetics and Molecular Biology (General), Genetics
CiteScore
1.60
自引率
0.00%
发文量
0
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


