Thin microporous polydimethylsiloxane membrane prepared by phase separation and its applications for cell culture
IF 3
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
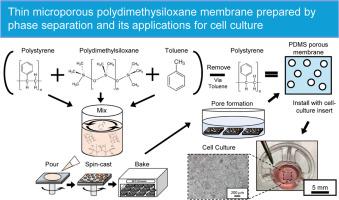
Animal experiments are often required for biological studies. However, in vitro cell culture models, such as cell-culture inserts and microphysiological systems, can provide a suitable alternative, making them essential tools in cell biology research, including the simulation of an organ environments closely related to the human body. Cell-culture inserts with porous membranes assist in recreating in vivo cell culture environments to study and process cell-culture assays. However, conventional cell culture membranes typically made of polyethylene terephthalate or polycarbonate cannot accommodate cell types that require deformable substrates. As such, this paper introduced a novel approach using spin-casting-assisted polymer-blend phase separation to create thin, flexible, and highly porous membranes for cell culture applications. Polydimethylsiloxane (PDMS) was selected as the material for the porous membrane, and polystyrene (PS) was used as a counter pair to induce phase separation with PDMS. PDMS facilitated the necessary reversible deformations during cell culture owing to its low elastic modulus. The thickness of the membrane and connectivity of the phase-separated PS domains can be adjusted, facilitating the fine-tuning of the pore size and density to improve the membrane performance. Therefore, this study successfully fabricated thin microporous PDMS membranes with improved performance over standard membranes for cell-culture inserts, namely a higher porosity, flexibility, and softness. The results of this study can enhance cell culture methodologies and contribute to a deeper understanding of cellular processes.
通过相分离制备的微孔聚二甲基硅氧烷薄膜及其在细胞培养中的应用
生物学研究通常需要动物实验。然而,体外细胞培养模型(如细胞培养插片和微生理系统)可以提供一种合适的替代方法,使其成为细胞生物学研究的重要工具,包括模拟与人体密切相关的器官环境。带有多孔膜的细胞培养插片有助于重现体内细胞培养环境,以研究和处理细胞培养试验。然而,传统的细胞培养膜通常由聚对苯二甲酸乙二醇酯或聚碳酸酯制成,无法适应需要可变形基底的细胞类型。因此,本文介绍了一种新方法,即利用自旋铸造辅助聚合物混合物相分离技术,为细胞培养应用制造出薄型、柔性和高多孔性膜。本文选择聚二甲基硅氧烷(PDMS)作为多孔膜的材料,并使用聚苯乙烯(PS)作为反相对来诱导与 PDMS 的相分离。由于 PDMS 的弹性模量较低,因此有利于细胞培养过程中必要的可逆变形。膜的厚度和相分离的 PS 域的连通性可以调整,从而有助于微调孔径和密度,提高膜的性能。因此,本研究成功制备出了微孔 PDMS 薄膜,与用于细胞培养插入物的标准膜相比,其性能得到了改善,即孔隙率更高、更灵活、更柔软。本研究的结果可改进细胞培养方法,有助于加深对细胞过程的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materialia
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
6.40
自引率
2.90%
发文量
345
审稿时长
36 days
期刊介绍:
Materialia is a multidisciplinary journal of materials science and engineering that publishes original peer-reviewed research articles. Articles in Materialia advance the understanding of the relationship between processing, structure, property, and function of materials.
Materialia publishes full-length research articles, review articles, and letters (short communications). In addition to receiving direct submissions, Materialia also accepts transfers from Acta Materialia, Inc. partner journals. Materialia offers authors the choice to publish on an open access model (with author fee), or on a subscription model (with no author fee).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


