Extraction of carbon and preparation of activated carbon from waste dry cell battery
IF 3.1
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
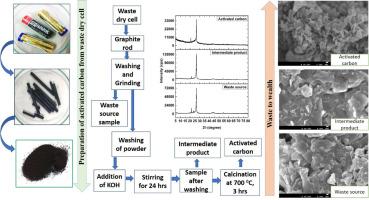
The goal of this research was to synthesize activated carbon (AC) from discarded batteries, and the crystallographic characterization of the final product (AC), intermediate product, and raw sources were explored. The formation of activated carbon was confirmed by utilizing an X-ray diffractometer (XRD) that revealed the structure of activated carbon was hexagonal. The crystallite size of activated carbon was computed by applying several model equations (Linear straight-line method of Scherrer's equation, Monshi-Scherrer method, Sahadat-Scherrer method, Size-Strain plot method, Halder-Wagner method, Williamson-Hall method), and the range of calculated crystallite size was 5–28 nm. The weight loss occurred in the two stages those was explored by a thermogravimetric analyzer (TGA). Fourier Transform Infrared Spectrophotometer (FTIR) confirmed that there was no significant change in the peak position between intermediate product and activated carbon except for the intensity and peak separation difference. Field Emission Electron Microscope (FE-SEM) revealed several types of shapes of the waste source, intermediate product, and main product (AC).
从废弃干电池中提取碳并制备活性炭
本研究的目标是利用废弃电池合成活性炭(AC),并探索最终产品(AC)、中间产品和原材料的晶体学特征。利用 X 射线衍射仪(XRD)确认了活性炭的形成,XRD 显示活性炭的结构为六角形。活性炭的结晶粒度是通过几个模型方程(舍勒方程直线法、Monshi-Scherrer 法、Sahadat-Scherrer 法、尺寸-应变图法、Halder-Wagner 法、Williamson-Hall 法)计算出来的,计算出的结晶粒度范围为 5-28 nm。热重分析仪(TGA)对两个阶段中发生的重量损失进行了分析。傅立叶变换红外分光光度计(FTIR)证实,中间产物和活性炭的峰值位置没有明显变化,只是强度和峰值分离度不同。场发射电子显微镜(FE-SEM)显示了废物源、中间产物和主要产物(AC)的几种形状。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbon Trends
Materials Science-Materials Science (miscellaneous)
CiteScore
4.60
自引率
0.00%
发文量
88
审稿时长
77 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


