Mitochondrial-uncoupling nanomedicine for self-heating and immunometabolism regulation in cancer cells
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Developing endogenous hyperthermia offers a promising strategy to address challenges with current exogenous hyperthermia techniques in clinics. Herein, a CD44-targeted and thermal-responsive nanocarrier was developed for the simultaneous delivery of 2,4-dinitrophenol and syrosingopine. The objective was to induce endogenous hyperthermia and regulate immunometabolism, ultimately augmenting anti-tumour immune responses. Dinitrophenol as mitochondrial uncoupler can convert electrochemical potential energy of inner mitochondrial membrane into heat, facilitating endogenous hyperthermia. Meanwhile, syrosingopine not only inhibits excessive lactate efflux caused by dinitrophenol but also downregulates tumour cell glycolysis, thus alleviating immunosuppression and heat shock protein (HSP)-dependent thermo-resistance through immunometabolism regulation. The synergistic effects of endogenous hyperthermia and immunometabolism regulation by this nanomedicine have potential to enhance tumor immunogenicity, reshape the tumour immune microenvironment, and effectively suppress the growth of subcutaneous tumours and patient-derived organoids in triple-negative breast cancer. Therefore, the endogenous hyperthermia strategy developed in this study would revolutionize hyperthermia for cancer treatment.
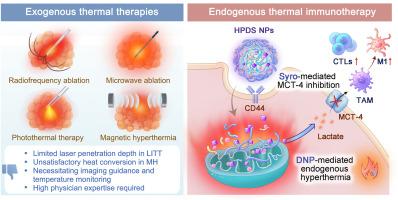
用于调节癌细胞自热和免疫代谢的线粒体耦合纳米药物
开发内源性热疗为解决目前临床上外源性热疗技术所面临的挑战提供了一种前景广阔的策略。本文开发了一种 CD44 靶向和热响应纳米载体,用于同时递送 2,4-二硝基苯酚和西罗星。其目的是诱导内源性高热和调节免疫代谢,最终增强抗肿瘤免疫反应。二硝基酚作为线粒体解偶联剂,可将线粒体内膜的电化学势能转化为热能,从而促进内源性高热。同时,西罗新碱不仅能抑制二硝基酚引起的乳酸过度外流,还能下调肿瘤细胞的糖酵解,从而通过免疫代谢调节减轻免疫抑制和热休克蛋白(HSP)依赖性热抵抗。这种纳米药物的内源性高热和免疫代谢调节的协同作用有望增强肿瘤的免疫原性,重塑肿瘤免疫微环境,并有效抑制三阴性乳腺癌皮下肿瘤和患者来源的器官组织的生长。因此,本研究开发的内源性热疗策略将彻底改变热疗在癌症治疗中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


