Supplementation of polyclonal antibodies, developed against epitope-string toxin-specific peptide immunogens, to commercial polyvalent antivenom, shows improved neutralization of Indian Big Four and Naja kaouthia snake venoms
IF 2.8
Q2 TOXICOLOGY
引用次数: 0
Abstract
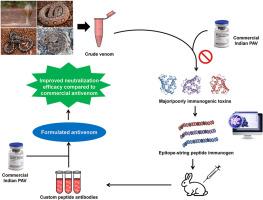
Snakebites profoundly impact the rural population of tropical nations, leading to significant socio-economic repercussions. Polyvalent antivenom (PAV) therapy faces several limitations, including intra-specific variations and poor efficacy against some major toxins and low molecular mass, poorly immunogenic toxins, which contribute to increased mortality and morbidity rates. Innovative strategies for developing novel antivenoms are continuously explored to address these challenges. The present study focuses on designing of 17 epitope-string toxin-specific peptide immunogens from pharmacologically active major and/or poorly immunogenic toxins (snake venom metalloprotease, Kunitz-type serine protease inhibitor, phospholipase A2, three-finger toxin) from the venom of the ‘Big Four’ venomous snakes and Naja kaouthia (NK) in India. These custom peptide antibodies demonstrated robust immuno-reactivity against the venoms ‘Big Four’ and NK. When these antibodies were supplemented with commercial PAV at a defined ratio (formulated polyvalent antivenom or FPAV), it significantly enhanced the neutralization of snake venom enzymes and in vivo neutralization of lethality and pharmacological activities such as haemorrhage, necrosis, pro-coagulant, defibrinogenation, and myotoxicity of ‘Big Four’ and NK venoms compared to PAV in mice. The present study highlights a promising strategy for developing next-generation antivenoms using synthetic peptide-based immunogens, offering a targeted approach to address the limitations of current antivenom therapy.
在商用多价抗蛇毒血清中添加针对表位串毒素特异性多肽免疫原开发的多克隆抗体,可提高对印度大四斑和 Naja kaouthia 蛇毒的中和效果
蛇咬伤对热带国家的农村人口造成了严重影响,导致了重大的社会经济后果。多价抗蛇毒血清(PAV)疗法面临着一些局限性,包括特异性内变异、对一些主要毒素和低分子质量、免疫原性差的毒素疗效不佳,这些都是导致死亡率和发病率上升的原因。为应对这些挑战,人们不断探索开发新型抗蛇毒血清的创新战略。本研究的重点是从印度 "四大毒蛇 "和 Naja kaouthia(NK)毒液中具有药理活性的主要和/或免疫原性差的毒素(蛇毒金属蛋白酶、Kunitz 型丝氨酸蛋白酶抑制剂、磷脂酶 A2、三指毒素)中设计出 17 种表位串毒素特异性多肽免疫原。这些定制的多肽抗体对 "四大毒蛇 "和 NK 毒液具有很强的免疫反应性。与 PAV 相比,当这些抗体按一定比例加入商用 PAV(配制多价抗蛇毒血清或 FPAV)时,可显著增强对蛇毒酶的中和作用,并在小鼠体内中和 "四大 "和 NK 毒液的致死性和药理活性,如出血、坏死、促凝血、去纤维蛋白原和肌毒性。本研究强调了利用基于合成肽的免疫原开发下一代抗蛇毒血清的前景广阔的战略,为解决目前抗蛇毒血清疗法的局限性提供了一种有针对性的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon: X
Pharmacology, Toxicology and Pharmaceutics-Toxicology
CiteScore
6.50
自引率
0.00%
发文量
33
审稿时长
14 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


