Interfacial Zn2+-solvation regulator towards reversible and stable Zn anode
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
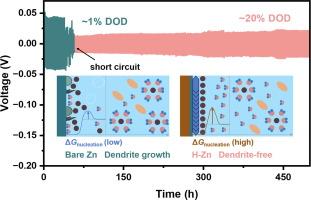
Aqueous zinc-ion batteries (AZIBs) are fundamentally challenged by the instability of the electrode/electrolyte interface, predominantly due to irreversible zinc (Zn) deposition and hydrogen evolution. Particularly, the intricate mechanisms behind the electrochemical discrepancies induced by interfacial Zn2+-solvation and deposition behavior demand comprehensive investigation. Organic molecules endowed with special functional groups (such as hydroxyl, carboxyl, etc.) have the potential to significantly optimize the solvation structure of Zn2+ and regulate the interfacial electric double layer (EDL). By increasing nucleation overpotential and decreasing interfacial free energy, these functional groups facilitate a lower critical nucleation radius, thereby forming an asymptotic nucleation model to promote uniform Zn deposition. Herein, this study presents a pioneering approach by introducing trace amounts of n-butanol as solvation regulators to engineer the homogenized Zn (H-Zn) anode with a uniform and dense structure. The interfacial reaction and structure evolution are explored by in/ex-situ experimental techniques, indicating that the H-Zn anode exhibits dendrite-free growth, no by-products, and weak hydrogen evolution, in sharp contrast to the bare Zn. Consequently, the H-Zn anode achieves a remarkable Zn utilization rate of approximately 20% and simultaneously sustains a prolonged cycle life exceeding 500 h. Moreover, the H-Zn//NH4V4O10 (NVO) full battery showcases exceptional cycle stability, retaining 95.04% capacity retention after 400 cycles at a large current density of 5 A g−1. This study enlightens solvation-regulated additives to develop Zn anode with superior utilization efficiency and extended operational lifespan.
实现可逆和稳定锌阳极的表面 Zn2+-溶解调节器
锌离子水电池(AZIBs)面临着电极/电解质界面不稳定的根本挑战,这主要是由于不可逆的锌(Zn)沉积和氢演化造成的。尤其需要全面研究界面 Zn2+ 溶解和沉积行为所引起的电化学差异背后的复杂机制。具有特殊官能团(如羟基、羧基等)的有机分子有可能显著优化 Zn2+ 的溶解结构并调节界面电双层(EDL)。通过提高成核过电位和降低界面自由能,这些官能团可降低临界成核半径,从而形成渐近成核模型,促进 Zn 的均匀沉积。本研究开创性地引入微量正丁醇作为溶解调节剂,设计出具有均匀致密结构的匀化锌(H-Zn)阳极。通过原位/离位实验技术对界面反应和结构演化进行了探索,结果表明 H-Zn 阳极呈现出无枝晶生长、无副产物和弱氢演化的特点,与裸锌形成鲜明对比。此外,H-Zn//NH4V4O10(NVO)全电池显示出卓越的循环稳定性,在 5 A g-1 的大电流密度下循环 400 次后仍能保持 95.04% 的容量。这项研究为开发具有更高的利用效率和更长的运行寿命的锌阳极提供了溶解调节添加剂的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


