Development of a Physiologically Based Pharmacokinetic (PBPK) Model for F-53B in Pregnant Mice and Its Extrapolation to Humans
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Chlorinated polyfluorinated ether sulfonic acid (F-53B), a commonly utilized alternative for perfluorooctane sulfonate, was detected in pregnant women and cord blood recently. However, the lack of detailed toxicokinetic information poses a significant challenge in assessing the human risk assessment for F-53B exposure. Our study aimed to develop a physiologically based pharmacokinetic (PBPK) model for pregnant mice, based on toxicokinetic experiments, and extrapolating it to humans. Pregnant mice were administered 80 μg/kg F-53B orally and intravenously on gestational day 13. F-53B concentrations in biological samples were analyzed via ultraperformance liquid chromatography–mass spectrometry. Results showed the highest F-53B accumulation in the brain, followed by the placenta, amniotic fluid, and liver in fetal mice. These toxicokinetic data were applied to F-53B PBPK model development and evaluation, and Monte Carlo simulations were used to characterize the variability and uncertainty in the human population. Most of the predictive values were within a 2-fold range of experimental data (>72%) and had a coefficient of determination (R2) greater than 0.68. The developed mouse model was then extrapolated to the human and evaluated with human biomonitoring data. Our study provides an important step toward improving the understanding of toxicokinetics of F-53B and enhancing the quantitative risk assessments in sensitive populations, particularly in pregnant women and fetuses.
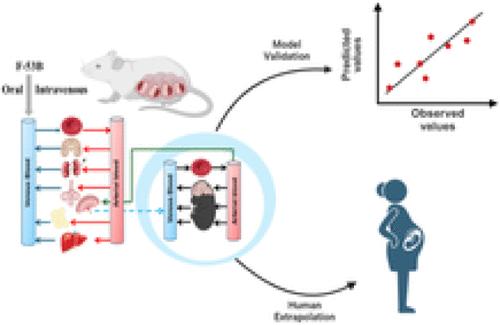
开发妊娠小鼠 F-53B 生理药代动力学 (PBPK) 模型并将其推广至人类
氯化多氟醚磺酸(F-53B)是一种常用的全氟辛烷磺酸替代品,最近在孕妇和脐带血中被检测到。然而,由于缺乏详细的毒物动力学信息,在评估人类接触 F-53B 的风险时面临巨大挑战。我们的研究旨在以毒物动力学实验为基础,为怀孕小鼠建立一个基于生理的药代动力学(PBPK)模型,并将其推断到人类。在妊娠第 13 天,给妊娠小鼠口服和静脉注射 80 μg/kg F-53B。生物样本中的 F-53B 浓度通过超高效液相色谱-质谱法进行了分析。结果显示,胎鼠大脑中的 F-53B 积累量最高,其次是胎盘、羊水和肝脏。这些毒物动力学数据被应用于 F-53B PBPK 模型的开发和评估,蒙特卡罗模拟被用于描述人类群体中的变异性和不确定性。大多数预测值都在实验数据的 2 倍范围内(72%),且决定系数 (R2) 大于 0.68。随后,我们将建立的小鼠模型推断到人类,并用人类生物监测数据进行了评估。我们的研究迈出了重要的一步,有助于加深对 F-53B 毒物代谢动力学的了解,加强对敏感人群(尤其是孕妇和胎儿)的定量风险评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


