Modulating membrane-bound enzyme activity with chemical stimuli
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Membrane-bound enzymes play pivotal roles in various cellular processes, making their activity regulation essential for cellular homeostasis and signaling transduction. Given that dysregulation of membrane-bound enzymes involved in various disease, controlling enzyme activity offers valuable avenues for designing targeted therapies and novel pharmaceutical interventions. This review explores chemical stimuli-responsive strategies for modulating the activity of these enzymes, employing diverse stimuli such as small molecules, proteins, nucleic acids, and bifunctional molecules to either inhibit or enhance their catalytic function. We systematically delineate the mechanisms underlying enzyme activity regulation, including substrate binding site blockade, conformational changes, and local concentration of enzymes and substrates. Furthermore, based on some examples, we elucidate the binding modalities between stimuli and enzymes, along with potential modes of regulation, and discuss their potential medical applications and future prospects. This review underscores the significance of understanding and manipulating enzyme activity on the cell membrane for advancing biomedical research and therapeutic development.
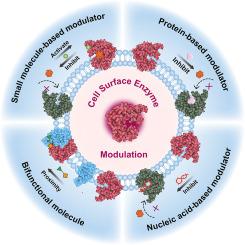
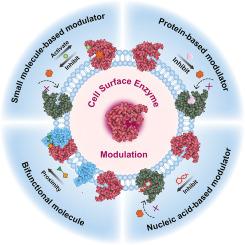
用化学刺激调节膜结合酶的活性
膜结合酶在各种细胞过程中发挥着关键作用,因此它们的活性调节对细胞的平衡和信号转导至关重要。鉴于膜结合酶的失调与各种疾病有关,控制酶的活性为设计靶向疗法和新型药物干预措施提供了宝贵的途径。本综述探讨了调节这些酶活性的化学刺激响应策略,利用小分子、蛋白质、核酸和双功能分子等各种刺激物来抑制或增强它们的催化功能。我们系统地阐述了酶活性调节的基本机制,包括底物结合位点阻断、构象变化以及酶和底物的局部浓度。此外,我们还根据一些实例,阐明了刺激物与酶之间的结合方式以及潜在的调节模式,并讨论了其潜在的医学应用和未来前景。这篇综述强调了了解和操纵细胞膜上酶的活性对于推进生物医学研究和治疗开发的重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


