PNIPAM Mesoglobules in Dependence on Pressure
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Poly(N-isopropylacrylamide) (PNIPAM) in aqueous solution forms mesoglobules above its cloud point temperature Tcp. While these are small and compact at atmospheric pressure, they are large and water-rich at high pressure. To identify the transition between these states, we employed optical microscopy and carried out isothermal pressure scans. Using very small angle neutron scattering, we determined the size and water content of the mesoglobules in pressure scans at different temperatures above Tcp. We observe a distinct transition at pressures of 35–55 MPa with the transition pressure depending on temperature. While the transition is smooth at high temperatures, i.e., far away from the coexistence line, it is abrupt at low temperatures, i.e., close to the coexistence line. Hence, at high temperatures, the swelling of the mesoglobules dominates, whereas at low temperatures, the coalescence of mesoglobules prevails. Subsequently decreasing the pressure results in a gradual deswelling of the mesoglobules at high temperature. In contrast, at low temperatures, small and compact mesoglobules form, but the large aggregates persist. We conclude that, on the time scale of the experiment, the disintegration of the large swollen aggregates into small and compact mesoglobules is only partially possible. Erasing the history by cooling the sample at the maximum pressure into the one-phase state does not result in qualitative changes for the behavior with the only difference that Fewer mesoglobules are formed when the pressure is decreased again. The newly identified transition line separates the low-pressure from the high-pressure regime.
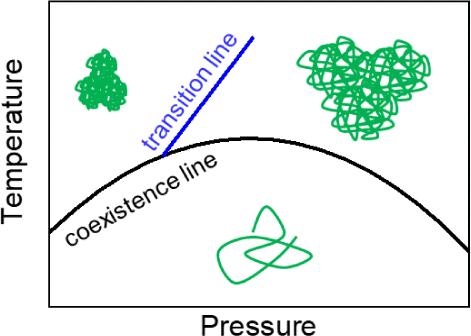
取决于压力的 PNIPAM 介质胶粒
水溶液中的聚(N-异丙基丙烯酰胺)(PNIPAM)会在其浊点温度 Tcp 以上形成中间球。在常压下,它们体积小、结构紧凑,而在高压下,它们体积大、富含水分。为了确定这些状态之间的转变,我们采用了光学显微镜并进行了等温压力扫描。利用极小角中子散射,我们确定了在高于 Tcp 的不同温度下进行压力扫描时介子球的大小和含水量。我们观察到在 35-55 兆帕压力下出现了明显的过渡,过渡压力取决于温度。在高温下,即远离共存线时,过渡是平滑的,而在低温下,即接近共存线时,过渡是突然的。因此,在高温下,中胶粒的膨胀占主导地位,而在低温下,中胶粒的凝聚占主导地位。随后降低压力会导致高温下的间球蛋白逐渐消胀。相反,在低温条件下,小而紧凑的间球体形成,但大的聚集体仍然存在。我们的结论是,在实验的时间尺度上,大的膨胀聚集体分解成小而紧密的间胶粒只有部分可能。通过在最大压力下将样品冷却到单相状态来消除历史记录,并不会导致行为发生质的变化,唯一的区别是,当压力再次降低时,形成的中胶粒更少。新发现的过渡线将低压和高压状态分开。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


