Utilizing l-Ascorbic Acid to Inhibit Adverse Oxidation and Enhance Denitration Efficiency during Sulfite Solution Absorption of NO2: Mechanism and Process Parameters
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
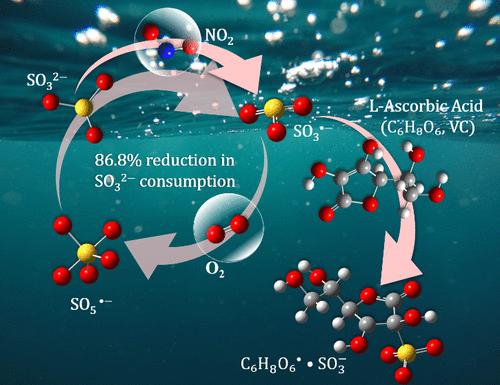
In the typical wet flue gas denitration technology using sulfite solutions, the rapid free radical chain reaction of SO3•– oxidation by O2 is the main reason for the significant consumption of absorbents and inefficient denitration. The study utilizes l-ascorbic acid (VC) to inhibit the free radical chain reaction. VC combined with SO3•– to form stable compounds, which hindered the oxidation of SO3•– to SO5•– by O2, leading to an 80.7 mol % decrease in Na2SO3 consumption by adding millimolar-level VC. Compared to the sulfite solution, the best denitration efficiency of the VC/Na2SO3 mixture solution increased from 94.4 to 99.2 vol %, and the absorption time for absorption efficiency above 90 vol % was extended from 7 to 72 min. Additionally, a macro absorption model describing the rates of macro absorption was established based on various operating conditions.
利用抗坏血酸抑制亚硫酸盐溶液吸收二氧化氮过程中的不利氧化并提高脱硝效率:机理与工艺参数
在使用亚硫酸盐溶液的典型湿法烟气脱硝技术中,SO3 被 O2 快速氧化的自由基连锁反应是造成大量吸收剂消耗和脱硝效率低下的主要原因。这项研究利用抗坏血酸(VC)来抑制自由基连锁反应。VC 与 SO3-- 结合形成稳定的化合物,阻碍了 O2 将 SO3-- 氧化成 SO5--,从而使加入毫摩尔级 VC 的 Na2SO3 消耗量减少了 80.7 摩尔%。与亚硫酸盐溶液相比,VC/Na2SO3 混合溶液的最佳脱硝效率从 94.4% 提高到 99.2%,吸收效率高于 90% 的吸收时间从 7 分钟延长到 72 分钟。此外,还根据不同的操作条件建立了描述宏观吸收率的宏观吸收模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
文献相关原料
公司名称
产品信息
阿拉丁
L-ascorbic acid
阿拉丁
Anhydrous sodium sulfite (Na2SO3)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


