Kinetic Laws of the Hydrochloric Acid Leaching of the Slime from Quartz–Leucoxene Sandstones
IF 0.4
Q4 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
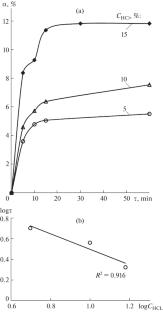
The kinetics of atmospheric hydrochloric acid leaching of the iron-containing slime formed during quartz–leucoxene sandstone beneficiation is studied. The calculated activation energies indicate two temperature ranges of the slime leaching: a diffusion kinetic region at 40–70°C (Ea = 35.79–36.56 kJ/mol) and a kinetic region at 70–80°C (Ea = 48.68 kJ/mol). A steplike character of the development of slime during the leaching of iron-containing minerals from it is revealed, which is caused by the consecutive processes of siderite and hematite decomposition and a change in the process rate as the solution is saturated with iron ions.
盐酸浸出石英褐煤砂岩粘泥的动力学规律
研究了石英-白蜡砂岩选矿过程中形成的含铁粘泥的常压盐酸浸出动力学。计算得出的活化能表明粘泥浸出有两个温度范围:40-70°C 的扩散动力学区(Ea = 35.79-36.56 kJ/mol)和 70-80°C 的动力学区(Ea = 48.68 kJ/mol)。在浸出含铁矿物的过程中,粘泥的发展呈现阶梯状,这是由于菱铁矿和赤铁矿的连续分解过程以及溶液中铁离子饱和时过程速率的变化造成的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Russian Metallurgy (Metally)
METALLURGY & METALLURGICAL ENGINEERING-
CiteScore
0.70
自引率
25.00%
发文量
140
期刊介绍:
Russian Metallurgy (Metally) publishes results of original experimental and theoretical research in the form of reviews and regular articles devoted to topical problems of metallurgy, physical metallurgy, and treatment of ferrous, nonferrous, rare, and other metals and alloys, intermetallic compounds, and metallic composite materials. The journal focuses on physicochemical properties of metallurgical materials (ores, slags, matters, and melts of metals and alloys); physicochemical processes (thermodynamics and kinetics of pyrometallurgical, hydrometallurgical, electrochemical, and other processes); theoretical metallurgy; metal forming; thermoplastic and thermochemical treatment; computation and experimental determination of phase diagrams and thermokinetic diagrams; mechanisms and kinetics of phase transitions in metallic materials; relations between the chemical composition, phase and structural states of materials and their physicochemical and service properties; interaction between metallic materials and external media; and effects of radiation on these materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


