Efficient Leaching Extraction of Vanadium Oxide from Spent Petrochemical Catalysts
IF 0.4
Q4 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
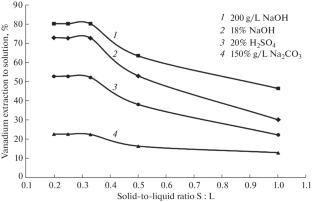
Abstract—The acidic, alkaline, and soda leaching of spent petrochemical catalysts to extract vanadium oxide is studied. The most efficient reagent, its consumption, leaching time, and temperature of the pulp are chosen using experimental results. The degree of extraction of vanadium oxide to the solution has been achieved at a level of 80% for a solution of sodium hydroxide (200 g/L) as a reagent, the solid-to-liquid ratio S : L = 1 : 3, a pulp temperature of 100°C, and a process time of about 3 h.
从废石化催化剂中高效浸出提取氧化钒
摘要 研究了酸性、碱性和苏打浸出废石化催化剂以提取氧化钒的方法。根据实验结果选择了最有效的试剂、试剂消耗量、浸出时间和矿浆温度。在氢氧化钠溶液(200 克/升)作为试剂、固液比 S : L = 1 : 3、纸浆温度为 100°C 和工艺时间约为 3 小时的条件下,溶液中氧化钒的萃取率达到了 80%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Russian Metallurgy (Metally)
METALLURGY & METALLURGICAL ENGINEERING-
CiteScore
0.70
自引率
25.00%
发文量
140
期刊介绍:
Russian Metallurgy (Metally) publishes results of original experimental and theoretical research in the form of reviews and regular articles devoted to topical problems of metallurgy, physical metallurgy, and treatment of ferrous, nonferrous, rare, and other metals and alloys, intermetallic compounds, and metallic composite materials. The journal focuses on physicochemical properties of metallurgical materials (ores, slags, matters, and melts of metals and alloys); physicochemical processes (thermodynamics and kinetics of pyrometallurgical, hydrometallurgical, electrochemical, and other processes); theoretical metallurgy; metal forming; thermoplastic and thermochemical treatment; computation and experimental determination of phase diagrams and thermokinetic diagrams; mechanisms and kinetics of phase transitions in metallic materials; relations between the chemical composition, phase and structural states of materials and their physicochemical and service properties; interaction between metallic materials and external media; and effects of radiation on these materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


