Synthesis and Phosphorylation of 5-Arylidenthiobarbiturates
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A series of 5-arylidene-2-thiobarbiturates was synthesized by Knoevenagel condensation from thiobarbituric acid under mild conditions; the use of water as a solvent allowed obtaining the target products in yields up to 78%. The addition reaction of dimethyl phosphite to 5-arylidene-2-thiobarbiturates was studied. The optimal conditions for this reaction were selected, and the high efficiency of cesium fluoride as catalyst was shown.
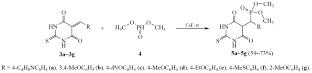
5-Arylidenthiobarbiturates 的合成与磷酸化
在温和的条件下,以硫代巴比妥酸为原料,通过克诺文纳格尔缩合反应合成了一系列 5-芳基-2-硫代巴比妥酸;以水为溶剂,目标产物的收率高达 78%。研究了亚磷酸二甲酯与 5-芳基-2-硫代巴比妥酸的加成反应。选择了该反应的最佳条件,并证明了氟化铯作为催化剂的高效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


