Synthesis and Alkylation of Pyrano[4′′,3′′:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidine-8,10-dithione
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Effective methods were proposed for the synthesis of 1-amino-8,8-dimethyl-5-piperidin-1-yl-8,9-dihydro-6H-pyrano[4,3-d]thieno[2,3-b]pyridin-2-carbonitrile and pyrano[4′′,3′′:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidine-8,10-dithione. A new intramolecular cyclization was discovered: the transformation of the thiazine ring into a pyrimidine ring. A plausible reaction mechanism was proposed, involving the formation of the corresponding thiazine ring followed by recyclization to the target compound. An effective method was developed for preparing bis-thioalkyl derivatives of thieno[3,2-d]pyrimidine with high yields.
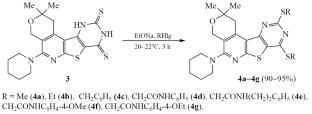
吡喃并[4′′,3′′:4′,5′]吡啶并[3′′,2′′:4,5]噻吩并[3,2-d]嘧啶-8,10-二硫酮的合成和烷基化反应
提出了合成 1-氨基-8,8-二甲基-5-哌啶-1-基-8,9-二氢-6H-吡喃并[4,3-d]噻吩并[2,3-b]吡啶-2-甲腈和吡喃并[4′′,3′′:4′,5′]pyrido[3′,2′:4,5]thieno[3,2-d]pyrimidine-8,10-dithione.发现了一种新的分子内环化现象:噻嗪环转变为嘧啶环。提出了一种合理的反应机理,包括形成相应的噻嗪环,然后再生成目标化合物。开发出一种有效的方法,可以高产率地制备噻吩并[3,2-d]嘧啶的双硫代烷基衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


