Surface freezing of cationic surfactant-adsorbed films at the oil-water interface: Impact on oil-in-water emulsion and pickering emulsion stability
IF 15.9
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
When n-alkanes or n-alcohols coexist with surfactants that have similar chain lengths, they can form mixed surface-frozen films at the oil-water interface. In this review, we first explain the basic characteristics of this surface freezing transition mainly from a thermodynamic viewpoint. Then, we discussed the effect of surface freezing of a cationic surfactant (cetyltrimethylammonium chloride: CTAC) with tetradecane, hexadecane, or hexadecanol on the kinetic stability of the oil-in-water (O/W) emulsions. We show that the surface frozen film not only increases the kinetic stability of the O/W emulsions but also stably encapsulates coexisting organic molecules in the oil core. Finally, we will introduce one of our recent works in which we observed that the exchange between silica nanoparticles and CTAC molecules occurs at the surface of Pickering emulsions when the oil-water interfacial tension is lowered by the surface freezing. The resulting detachment of silica particles from the oil-water interface broke the Pickering emulsion. The advantages of controlling the stability of O/W emulsions via the use of surface-frozen film are discussed in comparison with normal surfactant emulsifiers in the conclusion part of the review.
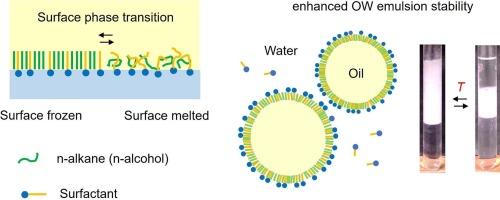
阳离子表面活性剂吸附膜在油水界面的表面冻结:对水包油乳液和酸洗乳液稳定性的影响。
当正构烷烃或正构醇与具有相似链长的表面活性剂共存时,它们会在油水界面形成混合表面冻结膜。在这篇综述中,我们首先主要从热力学角度解释了这种表面冻结转变的基本特征。然后,我们讨论了阳离子表面活性剂(十六烷基三甲基氯化铵:CTAC)与十四烷、十六烷或十六醇表面冻结对水包油(O/W)乳液动力学稳定性的影响。我们的研究表明,表面冷冻膜不仅能提高水包油型乳液的动力学稳定性,还能将共存的有机分子稳定地包裹在油芯中。最后,我们将介绍我们的一项最新研究成果,其中我们观察到,当表面冷冻降低油水界面张力时,硅纳米颗粒和 CTAC 分子之间的交换会在皮克林乳液表面发生。二氧化硅颗粒由此脱离油水界面,从而破坏了皮克林乳液。本综述的结论部分讨论了与普通表面活性剂乳化剂相比,通过使用表面冻结膜来控制 O/W 型乳液稳定性的优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.50
自引率
2.60%
发文量
175
审稿时长
31 days
期刊介绍:
"Advances in Colloid and Interface Science" is an international journal that focuses on experimental and theoretical developments in interfacial and colloidal phenomena. The journal covers a wide range of disciplines including biology, chemistry, physics, and technology.
The journal accepts review articles on any topic within the scope of colloid and interface science. These articles should provide an in-depth analysis of the subject matter, offering a critical review of the current state of the field. The author's informed opinion on the topic should also be included. The manuscript should compare and contrast ideas found in the reviewed literature and address the limitations of these ideas.
Typically, the articles published in this journal are written by recognized experts in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


