Fibrinogen αC-region acts as a functional safety latch: Implications for a fibrin biomechanical behaviour model
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
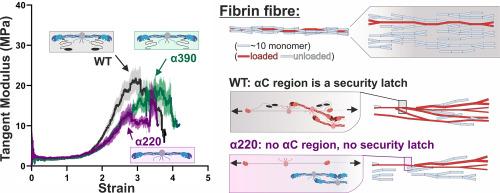
Fibrin has unique biomechanical properties which are essential for its role as a scaffold for blood clots. Fibrin is highly extensible and demonstrates significant strain stiffening behaviour, which is essential for stress-distribution in the network. Yet the exact structures of fibrin at the sub-fibre level that contribute to its unique biomechanical characteristic are unknown. Here we show how truncations of the fibrinogen αC-region impact the biomechanical properties of fibrin fibres. Surprisingly, absence of the complete αC-region did not influence the low strain modulus of fibrin fibres but led to premature fibre rupture and decreased extensibility. Intermediate effects were observed with partial deletion of the αC-region, reflected by intermediate rupture stress and toughness. However, overall strain-stiffening behaviour remained even in absence of the αC-region, indicating that strain stiffening is not due to stress being transferred from the αC-region to the protofibril backbone. Upon stress-relaxation, decay constants and their relative contribution to the total relaxation remained similar at all strains, showing that a distinct relaxation process is present until fibre rupture. However, relative contribution of fast relaxation was maximal only in crosslinked fibres if the flexible αC-connector was present. These data show that the αC-region is not the main load-bearing structure within fibrin fibres and point to a critical role for the protofibril backbone instead. We present a revised structural model based on protofibril branching that fully explains the unique biomechanical behaviour of fibrin fibres, while the αC-region primarily acts as a safety latch at the highest of strains.
Statement of significance
The findings presented in this paper reveal critically important details about how the molecular structure of fibrin contributes to its unique mechanical properties which are essential to fulfil its function as the scaffold of blood clots. In this work we used engineered proteins with alterations in an important but highly disordered area of the molecule called αC-region and we provide direct evidence for the first time for how the absence of either the globular αC-domain, or the complete αC-region impacts the mechanical behaviour of individual fibrin fibres. Using these results we developed a new structural model of protofibril organisation within fibrin fibres that fully explains their strain stiffening, relatively low modulus and their high, largely variable, extensibility.
纤维蛋白原 αC 区域充当功能安全锁:对纤维蛋白生物力学行为模型的影响
纤维蛋白具有独特的生物力学特性,这对其作为血凝块支架的作用至关重要。纤维蛋白具有高度延展性,并表现出显著的应变僵化行为,这对网络中的应力分布至关重要。然而,纤维蛋白在亚纤维水平上的确切结构对其独特的生物力学特性的贡献尚不清楚。在这里,我们展示了纤维蛋白原 αC 区域的截断如何影响纤维蛋白纤维的生物力学特性。令人惊讶的是,缺失完整的αC区并不会影响纤维蛋白纤维的低应变模量,但会导致纤维过早断裂并降低延伸性。部分缺失αC区会产生中间效应,表现为中间断裂应力和韧性。然而,即使没有αC区域,整体应变硬化行为仍然存在,这表明应变硬化并不是由于应力从αC区域转移到原纤维骨架。应力松弛时,衰减常数及其对总松弛的相对贡献在所有应变下都保持相似,这表明在纤维断裂之前存在一个独特的松弛过程。然而,只有在存在柔性αC连接体的交联纤维中,快速松弛的相对贡献才最大。这些数据表明,αC 区域并不是纤维蛋白纤维内的主要承载结构,而是原纤维骨架的关键作用。我们提出了一个基于原纤维分支的修正结构模型,该模型可充分解释纤维蛋白纤维的独特生物力学行为,而 αC 区域在最高应变时主要起安全锁扣的作用。意义说明:本文的研究结果揭示了纤维蛋白分子结构如何促成其独特机械特性的重要细节,而这些特性对于实现其作为血凝块支架的功能至关重要。在这项工作中,我们使用了在分子中一个重要但高度无序的区域(称为αC区)发生改变的工程蛋白,并首次提供了直接证据,证明球状αC区或完整αC区的缺失如何影响单个纤维蛋白纤维的机械性能。利用这些结果,我们建立了一个纤维蛋白纤维内原纤维组织的新结构模型,该模型可充分解释纤维蛋白纤维的应变僵化、相对较低的模量及其较高的、在很大程度上可变的延伸性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


