Histone lysine methylation modifiers controlled by protein stability
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
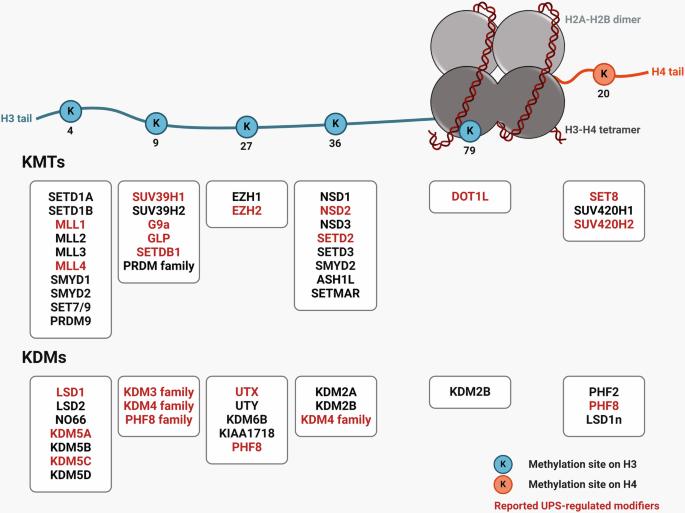
Histone lysine methylation is pivotal in shaping the epigenetic landscape and is linked to cell physiology. Coordination of the activities of multiple histone lysine methylation modifiers, namely, methyltransferases and demethylases, modulates chromatin structure and dynamically alters the epigenetic landscape, orchestrating almost all DNA-templated processes, such as transcription, DNA replication, and DNA repair. The stability of modifier proteins, which is regulated by protein degradation, is crucial for their activity. Here, we review the current knowledge of modifier-protein degradation via specific pathways and its subsequent impact on cell physiology through epigenetic changes. By summarizing the functional links between the aberrant stability of modifier proteins and human diseases and highlighting efforts to target protein stability for therapeutic purposes, we aim to promote interest in defining novel pathways that regulate the degradation of modifiers and ultimately increase the potential for the development of novel therapeutic strategies. Histone modifications, such as methylation, are key in controlling gene expression by changing the structure of chromatin, the DNA and protein mix in our cells’ nucleus. This study investigates how the stability of histone lysine methylation modifiers—enzymes that add or remove methyl groups from histones—is managed. It’s a study aimed at understanding the delicate balance of these modifiers in the cell. The researchers studied various cell processes, including the ubiquitin-proteasome system, and post-translational modifications, that affect the stability of these enzymes. The results show changing the stability of these modifiers can alter histone methylation patterns, suggesting new ways to target diseases like cancer. Researchers conclude that understanding the control of enzyme stability offers a promising path for developing therapies that can correct abnormal gene expression by targeting the enzymes responsible for histone modifications. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
由蛋白质稳定性控制的组蛋白赖氨酸甲基化修饰因子
组蛋白赖氨酸甲基化在形成表观遗传景观方面起着关键作用,并与细胞生理有关。多种组蛋白赖氨酸甲基化修饰因子(即甲基转移酶和去甲基化酶)的活动相互协调,可调节染色质结构,动态改变表观遗传学景观,协调几乎所有由 DNA 引发的过程,如转录、DNA 复制和 DNA 修复。修饰蛋白的稳定性受蛋白降解的调控,这对其活性至关重要。在这里,我们回顾了目前关于修饰蛋白通过特定途径降解及其随后通过表观遗传变化对细胞生理产生影响的知识。通过总结修饰蛋白稳定性异常与人类疾病之间的功能性联系,并重点介绍为治疗目的而针对蛋白稳定性所做的努力,我们希望提高人们对定义调节修饰蛋白降解的新途径的兴趣,并最终提高开发新型治疗策略的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


