The neuroendocrine transition in prostate cancer is dynamic and dependent on ASCL1
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Lineage plasticity is a hallmark of cancer progression that impacts therapy outcomes, yet the mechanisms mediating this process remain unclear. Here, we introduce a versatile in vivo platform to interrogate neuroendocrine lineage transformation throughout prostate cancer progression. Transplanted mouse prostate organoids with human-relevant driver mutations (Rb1−/−; Trp53−/−; cMyc+ or Pten−/−; Trp53−/−; cMyc+) develop adenocarcinomas, but only those with Rb1 deletion advance to aggressive, ASCL1+ neuroendocrine prostate cancer (NEPC) resistant to androgen receptor signaling inhibitors. Notably, this transition requires an in vivo microenvironment not replicated by conventional organoid culture. Using multiplexed immunofluorescence and spatial transcriptomics, we reveal that ASCL1+ cells arise from KRT8+ luminal cells, progressing into transcriptionally heterogeneous ASCL1+;KRT8− NEPC. Ascl1 loss in established NEPC causes transient regression followed by recurrence, but its deletion before transplantation abrogates lineage plasticity, resulting in castration-sensitive adenocarcinomas. This dynamic model highlights the importance of therapy timing and offers a platform to identify additional lineage plasticity drivers. Sawyers and colleagues describe an in vivo platform used to explore the dynamics and key factors of neuroendocrine lineage transformation. They find that Ascl1 depletion blocks plasticity and leads to cancer that is sensitive to castration.
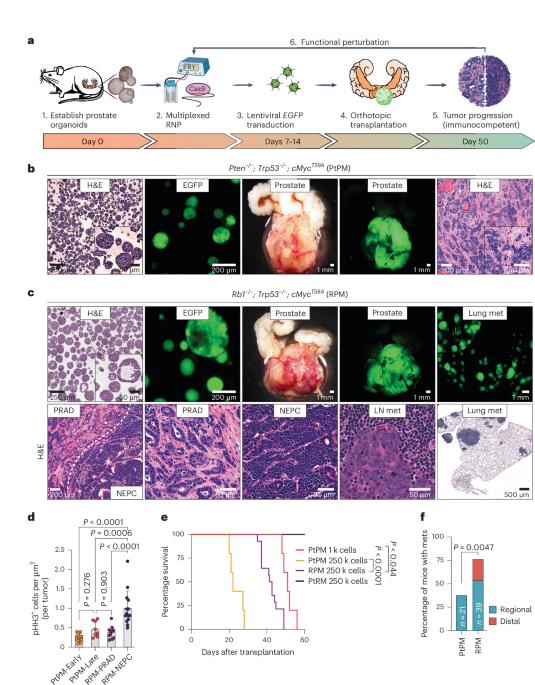
前列腺癌的神经内分泌转变是动态的,依赖于 ASCL1。
系谱可塑性是癌症进展的一个标志,会影响治疗效果,但这一过程的介导机制仍不清楚。在这里,我们引入了一个多功能体内平台来研究前列腺癌进展过程中的神经内分泌谱系转化。移植的小鼠前列腺器官组织具有人类相关的驱动突变(Rb1-/-; Trp53-/-; cMyc+或Pten-/-; Trp53-/-; cMyc+),会发展成腺癌,但只有Rb1缺失的组织才会发展成对雄激素受体信号抑制剂有抵抗力的侵袭性ASCL1+神经内分泌前列腺癌(NEPC)。值得注意的是,这种转变需要体内微环境,而传统的类器官培养无法复制。利用多重免疫荧光和空间转录组学,我们揭示了 ASCL1+ 细胞来源于 KRT8+ 管腔细胞,并发展成为转录异质性的 ASCL1+;KRT8- NEPC。在已建立的 NEPC 中缺失 Ascl1 会导致一过性消退,随后复发,但在移植前缺失 Ascl1 则会削弱细胞系的可塑性,导致对阉割敏感的腺癌。这一动态模型强调了治疗时机的重要性,并为确定其他细胞系可塑性驱动因素提供了一个平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


