New Advances in Understanding Inhibition of Myeloperoxidase and Neutrophil Serine Proteases by Two Families of Staphylococcal Innate Immune Evasion Proteins
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
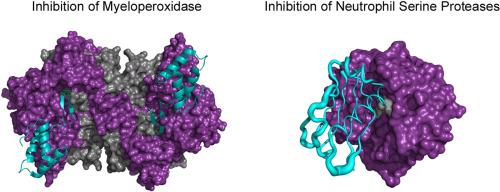
Neutrophils are the most abundant leukocytes in humans and play an important early role in the innate immune response against microorganisms. Neutrophil phagosomes contain high concentrations of antibacterial enzymes, including myeloperoxidase (MPO) and the neutrophil serine proteases (NSPs). These antibacterial enzymes can also be released extracellularly upon degranulation or as a component of neutrophil extracellular traps (NETs). Due to host/pathogen coevolution, S. aureus expresses a diverse arsenal of innate immune evasion proteins that target many aspects of the neutrophil antibacterial response. In the last decade, two new classes of staphylococcal innate immune evasion proteins that act as potent, selective inhibitors of MPO and NSPs, respectively, have been discovered. The Staphylococcal Peroxidase INhibitor (SPIN) is a small ∼8.3 kDa α-helical bundle protein that blocks MPO activity by interfering with substrate and product exchange with the MPO active site. The Extracellular Adherence Protein (EAP) family consists of three unique proteins comprised of one or more copies of an ∼11 kDa β-grasp domain capable of high-affinity, selective, non-covalent inhibition of NSPs. This brief review article summarizes recent advances in understanding the structural and functional properties of SPIN and EAP family members and outlines some potential avenues for future investigation of these enzyme inhibitors.
了解两个葡萄球菌先天性免疫规避蛋白家族抑制髓过氧化物酶和中性粒细胞丝氨酸蛋白酶的新进展
中性粒细胞是人体中数量最多的白细胞,在针对微生物的先天性免疫反应中发挥着重要的早期作用。中性粒细胞吞噬体中含有高浓度的抗菌酶,包括髓过氧化物酶(MPO)和中性粒细胞丝氨酸蛋白酶(NSPs)。这些抗菌酶也可在脱颗粒时释放到细胞外,或作为中性粒细胞胞外捕获物(NET)的组成部分释放出来。由于宿主/病原体的共同进化,金黄色葡萄球菌表达了多种多样的先天性免疫规避蛋白,这些蛋白针对中性粒细胞抗菌反应的许多方面。在过去十年中,人们发现了两类新的葡萄球菌先天免疫逃避蛋白,它们分别是 MPO 和 NSPs 的强效选择性抑制剂。葡萄球菌过氧化物酶抑制剂(SPIN)是一种小型的 ∼8.3 kDa α螺旋束蛋白,它通过干扰 MPO 活性位点的底物和产物交换来阻断 MPO 活性。细胞外粘附蛋白(EAP)家族由三种独特的蛋白组成,它们由一个或多个拷贝的 11 kDa β-抓取结构域组成,能够对 NSPs 进行高亲和力、选择性和非共价抑制。这篇简短的综述文章总结了在了解 SPIN 和 EAP 家族成员的结构和功能特性方面的最新进展,并概述了未来研究这些酶抑制剂的一些潜在途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


