Evaporation-Induced Switching from Flocculated to Dispersed TiO2 Nanoparticles in Binary Solvents
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Evaporation in mixed solutions containing both volatile and involatile solvents changes the properties of the binary solvent. We found that evaporating one solvent drastically increased the affinity of the mixed solvent to dispersant molecules and induced a switch from flocculated to dispersed TiO2 nanoparticles (TiO2NPs). We prepared dispersions of TiO2NPs with mixtures of volatile cyclosiloxane (Dx), involatile polar oil, and a dispersant, namely polyhydroxystearic acid (PHSA). Dx is a nonsolvent but the polar oil is a good solvent for PHSA. The dispersions were applied on a quartz substrate, and Dx was evaporated. The original applied films were turbid, in which flocculated TiO2NPs formed network structures. However, as the evaporation of Dx progressed, the drying films became transparent and the network structures of TiO2NPs loosened and disappeared. After the evaporation of Dx, the applied films were transparent to visible light but blocked the transmission of UV light. The flow characteristics of the dispersions also changed. The original dispersions showed shear-thinning but became more Newtonian-like as the fraction of Dx decreased. Generally, particles are concentrated in drying dispersions and become packed or flocculated as evaporation progresses. Our findings show that initially flocculated nanoparticles can be redispersed after evaporating a specific solvent. The effects of different Dxs and compositions on the switch from flocculated to dispersed TiO2NPs and their applicability as sunscreens are discussed.
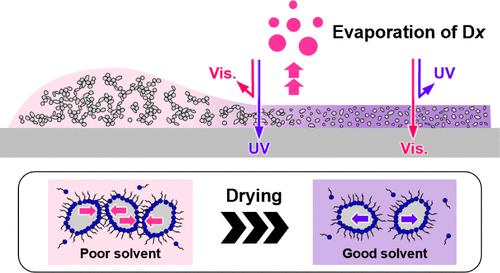
蒸发诱导二元溶剂中 TiO2 纳米粒子从絮凝到分散的转换
在含有挥发性和非挥发性溶剂的混合溶液中进行蒸发会改变二元溶剂的性质。我们发现,蒸发一种溶剂会大大增加混合溶剂对分散剂分子的亲和力,并促使 TiO2 纳米粒子(TiO2NPs)从絮凝状态转变为分散状态。我们用挥发性环硅氧烷(Dx)、不挥发性极性油和分散剂(即聚羟基硬脂酸(PHSA))的混合物制备了 TiO2NPs 分散液。Dx 是一种非溶剂,但极性油是 PHSA 的良好溶剂。将分散液涂在石英基底上,然后蒸发 Dx。最初涂抹的薄膜是浑浊的,其中絮凝的 TiO2NPs 形成了网络结构。然而,随着 Dx 蒸发的进行,干燥薄膜变得透明,TiO2NPs 的网络结构松散并消失。Dx 蒸发后,涂膜对可见光透明,但阻碍了紫外线的传输。分散体的流动特性也发生了变化。原来的分散体显示出剪切稀化,但随着 Dx 分数的减少,分散体变得更像牛顿流体。一般来说,颗粒会在干燥分散液中集中,并随着蒸发的进行而变得拥挤或絮凝。我们的研究结果表明,最初絮凝的纳米粒子可以在蒸发特定溶剂后重新分散。我们还讨论了不同的 Dx 和成分对 TiO2NPs 从絮凝到分散的转变的影响,以及它们作为防晒剂的适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


