Mass-Tagged Self-Assembled Nanoprobe Reveals the Transport of PD-L1 from Cancer Cells to Tumor-Educated Platelets
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
The expression level of immune checkpoint proteins detected by tissue biopsy is currently used as a predictive biomarker for immune checkpoint blockade (ICB) therapy. However, tissue biopsy is susceptible to invasive sample collection procedures, significant sampling heterogeneity, and the difficulty of repeated sampling. Therefore, liquid biopsy of blood samples is becoming an alternative choice for immune checkpoint protein detection. Among various vesicles in blood, platelets can obtain cancer information to form a specific group called tumor-educated platelets (TEPs). The platelet-derived proteins in TEPs may have a predictive potential in ICB therapy.Results
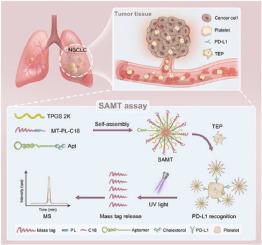
In this study, a photo-cleavable mass-tagged self-assembled (SAMT) nanoprobe with signal amplification was developed for the quantitative detection of PD-L1. The SAMT probe was assembled by photo-cleavable mass tags, PD-L1 aptamer, and amphiphilic polymer. After binding with PD-L1 on the platelet, the probe can release mass tags with UV light exposure. The amount of the mass tag, representing that of PD-L1, was subsequently determined by mass spectrometry. The assay sensitivity can be greatly improved by up to four orders of magnitude, achieving a detection limit of 10 fM. This assay was subsequently applied to cancer cells and platelet samples from non-small cell lung cancer (NSCLC) patients. The patients with higher tumor stages, higher degrees of lymph node invasion, and better ICB response had higher PD-L1 levels on platelets. Further investigation revealed that PD-L1 on the platelets was transported from cancer cells, providing evidence for the existence of TEPs.Significance
The SAMT probe can amplify the signal of the target molecule into that of multiple mass tags, achieving ultrasensitive ICB protein quantitative detection in platelets. Moreover, the employed SAMT assay not only revealed PD-L1 transport from cancer cells to platelets but also confirmed the presence of TEPs.
质量标记的自组装纳米探针揭示了 PD-L1 从癌细胞到肿瘤诱导血小板的迁移过程
背景通过组织活检检测免疫检查点蛋白的表达水平目前被用作免疫检查点阻断疗法(ICB)的预测性生物标记物。然而,组织活检容易受到侵入性样本采集程序、采样异质性大以及重复采样困难等因素的影响。因此,血液样本液体活检正成为免疫检查点蛋白检测的另一种选择。在血液中的各种囊泡中,血小板可以获取癌症信息,形成一个特定的群体,称为肿瘤教育血小板(TEPs)。结果在这项研究中,开发了一种具有信号放大功能的光可裂解质量标记自组装(SAMT)纳米探针,用于定量检测 PD-L1。SAMT 探针由光可裂解质量标签、PD-L1 合体和两亲聚合物组装而成。探针与血小板上的 PD-L1 结合后,可在紫外线照射下释放质量标签。质量标签的量代表 PD-L1 的量,随后通过质谱测定。检测灵敏度可提高四个数量级,达到 10 fM 的检测限。这种检测方法随后被应用于非小细胞肺癌(NSCLC)患者的癌细胞和血小板样本。肿瘤分期较高、淋巴结侵犯程度较高以及对 ICB 反应较好的患者血小板上的 PD-L1 水平较高。意义SAMT探针可将目标分子的信号放大为多个质量标签的信号,实现血小板中ICB蛋白的超灵敏定量检测。此外,所采用的 SAMT 检测方法不仅揭示了 PD-L1 从癌细胞向血小板的转运,还证实了 TEPs 的存在。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


