Fibroblast Growth Factor Receptor 1-Specific Dehydrogelation to Release Its Inhibitor for Enhanced Lung Tumor Therapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Fibroblast growth factor receptor 1 (FGFR1) is emerging as a promising molecular target of lung cancer, and various FGFR1 inhibitors have exhibited significant therapeutic effects on lung cancer in preclinical research. Due to their low targeting ability or bioavailability, direct administration of these inhibitors may cause side effects. Herein, a hydrogelator, Nap-Phe-Phe-Phe-Glu-Thr-Glu-Leu-Tyr-OH (Nap-Y), was rationally designed to coassemble with an FGFR1 inhibitor nintedanib (Nin) to form a peptide hydrogel Gel Y/Nin for localized administration and FGFR1-triggered release of Nin. Upon specific phosphorylation by FGFR1 overexpressed on lung cancer cells, Nap-Y in Gel Y/Nin is converted to the hydrophilic product Nap-Phe-Phe-Phe-Glu-Thr-Glu-Leu-Tyr(H2PO3)-OH (Nap-Yp), leading to dehydrogelation of the gel and subsequent Nin release. In vitro experiments demonstrate that the release of Nin in a sustained manner from Gel Y/Nin significantly suppresses the survival, migration, and invasion of A549 cells by inhibiting FGFR1 expression and its phosphorylation function on downstream signaling molecules. Nude mouse studies show that Gel Y/Nin exhibits enhanced therapeutic efficacy on lung tumor than free Nin. We anticipate that Gel Y/Nin will be utilized for lung cancer treatment in clinical settings in the near future.
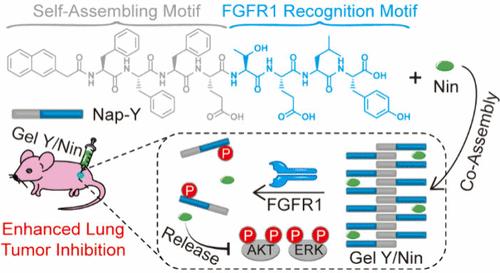
成纤维细胞生长因子受体 1 特异性脱水凝胶技术释放其抑制剂以加强肺癌治疗
成纤维细胞生长因子受体 1(FGFR1)正逐渐成为肺癌的一个有希望的分子靶点,在临床前研究中,各种 FGFR1 抑制剂已显示出对肺癌的显著治疗效果。由于这些抑制剂的靶向能力或生物利用度较低,直接给药可能会产生副作用。在此,我们合理地设计了一种水凝胶剂--Nap-Phe-Phe-Glu-Thr-Glu-Leu-Tyr-OH(Nap-Y),它能与表皮生长因子受体1抑制剂宁替尼(Nin)共同组装成一种多肽水凝胶 Gel Y/Nin,用于局部给药和表皮生长因子受体1触发的 Nin 释放。在肺癌细胞上过表达的 FGFR1 发生特异性磷酸化后,Gel Y/Nin 中的 Nap-Y 转化为亲水性产物 Nap-Phe-Phe-Glu-Thr-Glu-Leu-Tyr(H2PO3)-OH(Nap-Yp),导致凝胶脱水凝胶化,随后释放 Nin。体外实验表明,凝胶 Y/Nin 通过抑制表皮生长因子受体 1(FGFR1)的表达及其对下游信号分子的磷酸化功能,以持续的方式释放 Nin,从而显著抑制了 A549 细胞的存活、迁移和侵袭。裸鼠研究表明,与游离 Nin 相比,Gel Y/Nin 对肺肿瘤的疗效更佳。我们预计在不久的将来,Gel Y/Nin 将被用于肺癌的临床治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


