Single-Atom Electrocatalysts for Water Splitting in Acidic Media
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The proton exchange membrane water electrolyzer (PEMWE) is regarded as the most promising technique to convert intermittent renewable energy sources into clean and storable hydrogen through electrocatalytic water splitting. However, commercial electrocatalysts for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) are based on expensive platinum group metals (PGMs), which predominantly hinder the large-scale application of PEMWE. Single-atom electrocatalysts (SAECs) with atomic level dispersion of metal active sites can greatly minimize the usage amount of precious metals while keeping the efficient electrocatalytic activities. These advantages make SAECs attractive for their application in PEMWE. In this review, the mechanism of the HER and OER, together with general synthesis strategies of SAECs, was introduced and discussed. Subsequently, the recent development of SAECs based on (non)precious metals for acidic HER, OER, and overall water splitting is summarized, highlighted with the mechanism understanding between the electronic structure and electrocatalytic performance. Finally, the challenges and perspectives of SAECs for acidic water splitting are proposed.
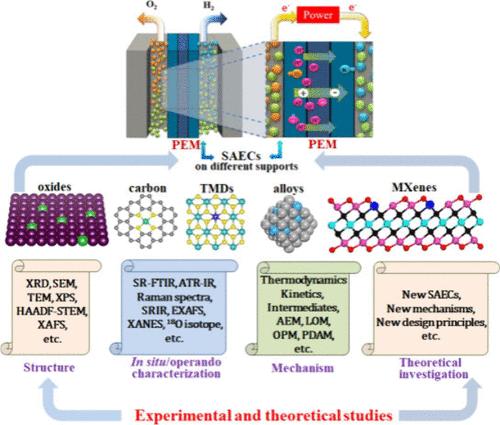
在酸性介质中进行水分离的单原子电催化剂
质子交换膜水电解槽(PEMWE)被认为是通过电催化水分裂将间歇性可再生能源转化为清洁和可储存氢气的最有前途的技术。然而,用于氢进化反应(HER)和氧进化反应(OER)的商用电催化剂基于昂贵的铂族金属(PGM),这主要阻碍了 PEMWE 的大规模应用。单原子电催化剂(SAECs)具有原子级分散的金属活性位点,可在保持高效电催化活性的同时大大减少贵金属的用量。这些优势使得单原子电催化剂在 PEMWE 中的应用极具吸引力。本综述介绍并讨论了 HER 和 OER 的机理以及 SAECs 的一般合成策略。随后,总结了基于(非)贵金属的 SAECs 在酸性 HER、OER 和整体水分离方面的最新发展,重点介绍了电子结构与电催化性能之间的机理。最后,提出了用于酸性水分离的 SAECs 所面临的挑战和前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


