Accuracy evaluation of Roche Accu-Chek Performa blood glucose meters at low glucose concentrations: A nine-year retrospective study
Abstract
Objective
To evaluate the accuracy of Roche Accu-Chek Performa glucose meters at a low glucose concentration of <5.55 mmol/L (100 mg/dL) over a 9-year period.
Methods
The accuracy of the Roche Accu-Chek Performa glucose meters at low glucose concentrations was evaluated using annual comparison data for 9 consecutive years from 2015 to 2023, according to the acceptability criteria specified in International Organization for Standardization (ISO) 15197:2013. Blood samples with low glucose concentrations of <5.55 mmol/L were prepared by incubation and glycolysis. The glucose concentration was detected using Roche Accu-Chek Performa glucose meters and a biochemical analyzer in the central laboratory.
Results
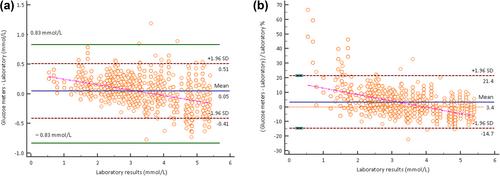
A total of 2978 pairs of comparison results from 211 glucose meters at a low glucose concentration of <5.55 mmol/L were retrospectively analyzed from 2015 to 2023. The clinical use duration spanned from 1 to 9 years and 40.76% (86 out of 211 glucose meters) had been used for more than 2 years. The correlation coefficient r between glucose meter measurements and laboratory reference values was 0.98 (p < 0.001). The mean according to Roche Accu-Chek Performa glucose meters was 0.05 mmol/L (0.9 mg/dL) higher than that of the biochemical analyzer (Z = −13.82, p < 0.0001). The results showed that 100.00% (211 out of 211) of the Roche Accu-Chek Performa glucose meters met the acceptability criteria specified in ISO 15197:2013. At a low glucose concentration of <5.55 mmol/L, 99.90% (2975 out of 2978) of the comparative data pairs in the error distribution fell within the range of ±0.83 mmol/L (15 mg/dL). Parkes consensus error grid analysis showed that 100.00% (2978 out of 2978) of comparative data pairs fell within region A.
Conclusions
This study demonstrated that Roche Accu-Chek Performa glucose meters successfully met the accuracy standards of ISO 15197:2013 for measuring blood glucose within the hypoglycemic range. Greater attention should be given to the performance of blood glucose monitoring systems in the low glycemic range, especially for patients with diabetes who are prone to hypoglycemia and require precise measurements.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


