Surrounding tissue morphogenesis with disrupted posterior midgut invagination during Drosophila gastrulation
IF 2.5
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Gastrulation involves multiple, physically-coupled tissue rearrangements. During Drosophila gastrulation, posterior midgut (PMG) invagination promotes both germband extension and hindgut invagination, but whether the normal epithelial rearrangement of PMG invagination is required for morphogenesis of the connected tissues has been unclear. In steppke mutants, epithelial organization of the PMG primordium is strongly disrupted. Despite this disruption, germband extension and hindgut invagination are remarkably effective, and involve myosin network inductions known to promote their wild-type remodelling. Known tissue-autonomous signaling could explain the planar-polarized, junctional myosin networks of the germband, but pushing forces from PMG invagination have been implicated in inducing apical myosin networks of the hindgut primordium. To confirm that the wave of hindgut primordium myosin accumulations is due to mechanical effects, rather than diffusive signalling, we analyzed α-catenin RNAi embryos, in which all of the epithelial tissues initially form but then lose cell-cell adhesion, and observed strongly diminished hindgut primordium myosin accumulations. Thus, alternate mechanical changes in steppke mutants seem to circumvent the lack of normal PMG invagination to induce hindgut myosin networks and invagination. Overall, both germband extension and hindgut invagination are robust to experimental disruption of the PMG invagination, and, although the processes occur with some abnormalities in steppke mutants, there is remarkable redundancy in the multi-tissue system. Such redundancy could allow complex morphogenetic processes to change over evolutionary time.
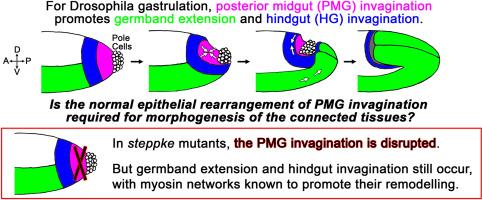
果蝇胃形成过程中后部中肠内陷的周围组织形态发生紊乱
胃形成涉及多种物理耦合的组织重排。在果蝇的胃形成过程中,后中肠(PMG)内陷同时促进胚芽带延伸和后肠内陷,但PMG内陷的正常上皮重排是否是相连组织形态发生所必需的一直不清楚。在steppke突变体中,PMG原基的上皮组织被严重破坏。尽管发生了这种破坏,胚芽带延伸和后肠内陷仍然非常有效,并且涉及已知能促进其野生型重塑的肌球蛋白网络诱导。已知的组织自主信号传导可以解释胚芽带的平面极化、交界肌球蛋白网络,但PMG内陷产生的推力与诱导后肠原基顶端肌球蛋白网络有关。为了证实后肠原基肌球蛋白聚集波是由机械效应而非扩散信号引起的,我们对α-catenin RNAi胚胎进行了分析,在这种胚胎中,所有上皮组织最初都已形成,但随后失去了细胞-细胞粘连,我们观察到后肠原基肌球蛋白聚集明显减少。因此,steppke突变体中的交替机械变化似乎可以规避正常PMG内陷的缺乏,从而诱导后肠肌球蛋白网络和内陷。总之,胚芽带伸展和后肠内陷对试验性破坏永磁发电机内陷都很稳健,尽管在steppke突变体中这些过程出现了一些异常,但在多组织系统中存在显著的冗余性。这种冗余性可以使复杂的形态发生过程随着进化时间而改变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


