Impact of the local renin–angiotensin system in perivascular adipose tissue on vascular health and disease
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Perivascular adipose tissue (PVAT) is found locally around blood vessels. It has the ability to release vasoactive chemicals, such as factors that relax and contract blood vessels. PVAT is now recognized as an endocrine organ with metabolic activity and as a “protagonist” for maintaining vascular homeostasis. Angiotensin II, a powerful vasoconstrictor of the renin–angiotensin system (RAS) that can increase blood pressure and vascular tone, is produced locally by PVAT. To mitigate the multiple vascular effects of angiotensin II, PVAT also generates molecules such as angiotensin (1–7), adiponectin, and nitric oxide. Reactive oxygen species and proinflammatory cytokines are produced in greater amounts when PVAT-mediated angiotensin II is present, resulting in endothelial dysfunction, inflammation, atherosclerosis, and other vascular disorders. The anticontractile and procontractile nature of PVAT is frequently disrupted in obese individuals, which increases the production of angiotensin II and decreases the production of anti-inflammatory and vasodilatory factors. These changes in turn exacerbate vascular inflammation, hypertension, and atherosclerosis. PVAT, which is crucial for maintaining vascular homeostasis, loses its anticontractile effect in obesity due to adipocyte hypertrophy, inflammation, and oxidative stress, exacerbating endothelial dysfunction. Overactive RAS in PVAT facilitates the migration and proliferation of vascular smooth muscle cells and atherosclerotic plaques, both of which accelerate the development of atherosclerosis. Targeting PVAT and the local RAS can offer therapeutic benefits in treating hypertension, atherosclerosis, and other vascular diseases. This review highlights the scientific underpinnings of the function of PVAT in regulating the autocrine and paracrine activities of vascular RAS constituents.
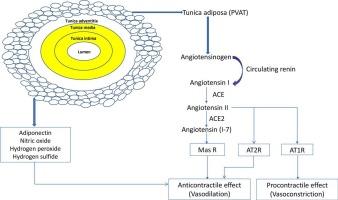
血管周围脂肪组织中的局部肾素-血管紧张素系统对血管健康和疾病的影响。
血管周围脂肪组织(PVAT)位于血管周围。它能够释放具有血管活性的化学物质,如放松和收缩血管的因子。目前,人们已认识到血管周围脂肪组织是一个具有新陈代谢活性的内分泌器官,也是维持血管平衡的 "主角"。血管紧张素 II 是肾素-血管紧张素系统(RAS)的一种强效血管收缩剂,可升高血压和血管张力,由 PVAT 在局部产生。为了减轻血管紧张素 II 对血管的多重影响,PVAT 还会产生血管紧张素 (1-7)、脂联素和一氧化氮等分子。当 PVAT 介导的血管紧张素 II 存在时,会产生更多的活性氧和促炎细胞因子,导致内皮功能障碍、炎症、动脉粥样硬化和其他血管疾病。肥胖者的 PVAT 的抗收缩和促收缩特性经常受到破坏,这增加了血管紧张素 II 的产生,减少了抗炎因子和血管舒张因子的产生。这些变化反过来又加剧了血管炎症、高血压和动脉粥样硬化。肥胖症患者的脂肪细胞肥大、炎症和氧化应激使对维持血管平衡至关重要的 PVAT 失去了抗收缩作用,从而加剧了内皮功能障碍。PVAT 中过度活跃的 RAS 会促进血管平滑肌细胞和动脉粥样硬化斑块的迁移和增殖,这两者都会加速动脉粥样硬化的发展。以 PVAT 和局部 RAS 为靶点可为治疗高血压、动脉粥样硬化和其他血管疾病带来疗效。本综述重点介绍了 PVAT 在调节血管 RAS 成分的自分泌和旁分泌活动方面的功能的科学依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


