Discovery of Benzo[d]oxazoles as Novel Dual Small-Molecule Inhibitors Targeting PD-1/PD-L1 and VISTA Pathway
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
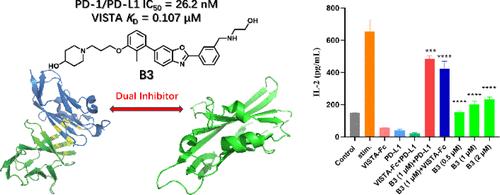
The blockers of programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) pathway have achieved great clinical success. However, the limited efficacy and low tumor response rate of anti-PD-1/PD-L1 monotherapy limit the clinical application of PD-1/PD-L1 inhibitors. V-domain immunoglobulin suppressor of T-cell activation (VISTA), a novel checkpoint regulator, exhibits potential synergy with PD-1/PD-L1 in enhancing antitumor immunity. Herein, we report the discovery of benzo[d]oxazole B3 as novel dual small-molecule inhibitors targeting PD-1/PD-L1 and VISTA with high PD-1/PD-L1 inhibitory activity and VISTA binding affinity. B3 rescues the immunosuppression of T-cells mediated by PD-L1 and VISTA and activates antitumor immunity effectively. Moreover, B3 could induce degradation of PD-L1 and VISTA in tumor cell. Furthermore, B3 displays significant in vivo antitumor efficacy in a CT26 mouse model. Our results discover B3 as a promising dual PD-1/PD-L1 and VISTA inhibitor, providing a novel therapeutic strategy to overcome the limitations of current anti-PD-1/PD-L1 therapy.
发现以 PD-1/PD-L1 和 VISTA 通路为靶点的苯并[d]恶唑类新型双重小分子抑制剂
程序性细胞死亡-1(PD-1)/程序性细胞死亡-配体1(PD-L1)通路阻断剂在临床上取得了巨大成功。然而,抗PD-1/PD-L1单药治疗的有限疗效和较低的肿瘤反应率限制了PD-1/PD-L1抑制剂的临床应用。V域免疫球蛋白T细胞活化抑制因子(VISTA)是一种新型检查点调节因子,在增强抗肿瘤免疫力方面与PD-1/PD-L1具有潜在的协同作用。在此,我们报告了苯并[d]恶唑 B3 的发现,它是针对 PD-1/PD-L1 和 VISTA 的新型双重小分子抑制剂,具有很高的 PD-1/PD-L1 抑制活性和 VISTA 结合亲和力。B3 能解除 PD-L1 和 VISTA 对 T 细胞的免疫抑制,有效激活抗肿瘤免疫。此外,B3 还能诱导肿瘤细胞中 PD-L1 和 VISTA 的降解。此外,B3 在 CT26 小鼠模型中显示出显著的体内抗肿瘤疗效。我们的研究结果发现,B3是一种很有前景的PD-1/PD-L1和VISTA双重抑制剂,为克服目前抗PD-1/PD-L1疗法的局限性提供了一种新的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


