ZeroCAL: Eliminating Carbon Dioxide Emissions from Limestone’s Decomposition to Decarbonize Cement Production
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Limestone (calcite, CaCO3) is an abundant and cost-effective source of calcium oxide (CaO) for cement and lime production. However, the thermochemical decomposition of limestone (∼800 °C, 1 bar) to produce lime (CaO) results in substantial carbon dioxide (CO2(g)) emissions and energy use, i.e., ∼1 tonne [t] of CO2 and ∼1.4 MWh per t of CaO produced. Here, we describe a new pathway to use CaCO3 as a Ca source to make hydrated lime (portlandite, Ca(OH)2) at ambient conditions (p, T)─while nearly eliminating process CO2(g) emissions (as low as 1.5 mol. % of the CO2 in the precursor CaCO3, equivalent to 9 kg of CO2(g) per t of Ca(OH)2)─within an aqueous flow-electrolysis/pH-swing process that coproduces hydrogen (H2(g)) and oxygen (O2(g)). Because Ca(OH)2 is a zero-carbon precursor for cement and lime production, this approach represents a significant advancement in the production of zero-carbon cement. The Zero CArbon Lime (ZeroCAL) process includes dissolution, separation/recovery, and electrolysis stages according to the following steps: (Step 1) chelator (e.g., ethylenediaminetetraacetic acid, EDTA)-promoted dissolution of CaCO3 and complexation of Ca2+ under basic (>pH 9) conditions, (Step 2a) Ca enrichment and separation using nanofiltration (NF), which allows separation of the Ca-EDTA complex from the accompanying bicarbonate (HCO3–) species, (Step 2b) acidity-promoted decomplexation of Ca from EDTA, which allows near-complete chelator recovery and the formation of a Ca-enriched stream, and (Step 3) rapid precipitation of Ca(OH)2 from the Ca-enriched stream using electrolytically produced alkalinity. These reactions can be conducted in a seawater matrix yielding coproducts including hydrochloric acid (HCl) and sodium bicarbonate (NaHCO3), resulting from electrolysis and limestone dissolution, respectively. Careful analysis of the reaction stoichiometries and energy balances indicates that approximately 1.35 t of CaCO3, 1.09 t of water, 0.79 t of sodium chloride (NaCl), and ∼2 MWh of electrical energy are required to produce 1 t of Ca(OH)2, with significant opportunity for process intensification. This approach has major implications for decarbonizing cement production within a paradigm that emphasizes the use of existing cement plants and electrification of industrial operations, while also creating approaches for alkalinity production that enable cost-effective and scalable CO2 mineralization via Ca(OH)2 carbonation.
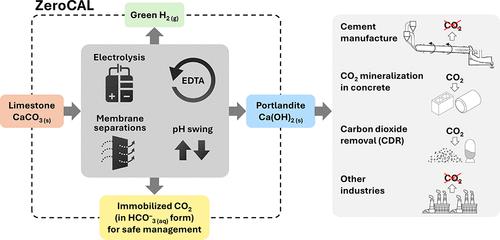
ZeroCAL:消除石灰石分解产生的二氧化碳排放,实现水泥生产的低碳化
石灰石(方解石,CaCO3)是水泥和石灰生产所需的氧化钙(CaO)的丰富且具有成本效益的来源。然而,石灰石热化学分解(800 °C,1 巴)生产石灰(CaO)会产生大量二氧化碳(CO2(g))排放和能源消耗,即每生产 1 吨 CaO 会产生 1 吨二氧化碳(CO2)和 1.4 兆瓦时(MWh)。在这里,我们描述了一种新的途径,在环境条件(p, T)下使用 CaCO3 作为 Ca 源来制造熟石灰(硅酸盐,Ca(OH)2),同时几乎消除了工艺过程中的 CO2(g) 排放(低至 1.5 mol. %,相当于每吨 Ca(OH)2(9 千克 CO2(g)))--在水流电解/pH 波动过程中同时产生氢气 (H2(g)) 和氧气 (O2(g))。由于 Ca(OH)2是水泥和石灰生产中的零碳前驱体,这种方法代表了零碳水泥生产的重大进步。零碳石灰(ZeroCAL)工艺包括溶解、分离/回收和电解阶段,具体步骤如下:(步骤 1)螯合剂(例如步骤 1)螯合剂(如乙二胺四乙酸,EDTA)促进 CaCO3 的溶解,并在碱性(>;步骤 2a)使用纳滤 (NF) 富集和分离 Ca,使 Ca-EDTA 复合物与伴随的碳酸氢盐 (HCO3-) 物种分离;(步骤 2b)酸性促进 Ca 与 EDTA 的解络合,使螯合剂接近完全回收并形成富 Ca 流;以及(步骤 3)使用电解产生的碱度从富 Ca 流中快速沉淀 Ca(OH)2。这些反应可在海水基质中进行,产生的副产品包括盐酸 (HCl) 和碳酸氢钠 (NaHCO3),分别来自电解和石灰石溶解。对反应化学计量学和能量平衡的仔细分析表明,生产 1 吨 Ca(OH)2 大约需要 1.35 吨 CaCO3、1.09 吨水、0.79 吨氯化钠 (NaCl),以及 2 兆瓦时的电能,这为强化工艺提供了很大的机会。这种方法对于在强调利用现有水泥厂和工业运营电气化的模式下实现水泥生产脱碳具有重大意义,同时还能创造出碱度生产方法,通过 Ca(OH)2 碳化实现具有成本效益和可扩展的二氧化碳矿化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


