Discovery of Propionic Acid Derivatives with a 5-THIQ Core as Potent and Orally Bioavailable Keap1–Nrf2 Protein–Protein Interaction Inhibitors for Acute Kidney Injury
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Keap1 plays a crucial role in regulating the Nrf2-mediated cytoprotective response and is increasingly targeted for oxidative stress-related diseases. Using small molecules to disrupt the Keap1–Nrf2 protein–protein interaction (PPI) has emerged as a new strategy for developing Nrf2 activators. Through extensive structure–activity relationship studies, we identified compound 56, which features a unique 5-tetrahydroisoquinoline scaffold and acts as a potent inhibitor of the Keap1–Nrf2 PPI. Compound 56 exhibited significant inhibitory activity (IC50 = 16.0 nM) and tight Keap1 binding affinity (Kd = 3.07 nM), along with acceptable oral bioavailability (F = 20%). Notably, 56 enhanced antioxidant defenses in HK-2 renal tubular epithelial cells and significantly reduced plasma creatinine and blood urea nitrogen levels in acute kidney injury (AKI) mice. These findings collectively position compound 56 as a promising candidate for the treatment of AKI.
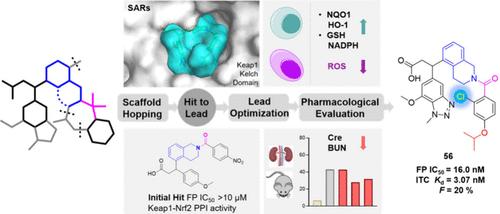
发现以 5-THIQ 为核心的丙酸衍生物,作为强效口服生物活性 Keap1-Nrf2 蛋白-蛋白相互作用抑制剂治疗急性肾损伤
Keap1 在调节 Nrf2 介导的细胞保护反应中发挥着至关重要的作用,并日益成为氧化应激相关疾病的靶向药物。利用小分子破坏 Keap1-Nrf2 蛋白-蛋白相互作用(PPI)已成为开发 Nrf2 激活剂的一种新策略。通过广泛的结构-活性关系研究,我们发现了化合物 56,它具有独特的 5-四氢异喹啉支架,是 Keap1-Nrf2 PPI 的强效抑制剂。化合物 56 具有明显的抑制活性(IC50 = 16.0 nM)和与 Keap1 紧密结合的亲和力(Kd = 3.07 nM),同时具有可接受的口服生物利用度(F = 20%)。值得注意的是,56 能增强 HK-2 肾小管上皮细胞的抗氧化防御能力,并显著降低急性肾损伤(AKI)小鼠的血浆肌酐和血尿素氮水平。这些发现共同将 56 号化合物定位为治疗 AKI 的有望候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


