Hepatic FXR-FGF4 is required for bile acid homeostasis via an FGFR4-LRH-1 signal node under cholestatic stress
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Bile acid (BA) homeostasis is vital for various physiological processes, whereas its disruption underlies cholestasis. The farnesoid X receptor (FXR) is a master regulator of BA homeostasis via the ileal fibroblast growth factor (FGF)15/19 endocrine pathway, responding to postprandial or abnormal transintestinal BA flux. However, the de novo paracrine signal mediator of hepatic FXR, which governs the extent of BA synthesis within the liver in non-postprandial or intrahepatic cholestatic conditions, remains unknown. We identified hepatic Fgf4 as a direct FXR target that paracrinally signals to downregulate Cyp7a1 and Cyp8b1. The effect of FXR-FGF4 is mediated by an uncharted intracellular FGF receptor 4 (FGFR4)-LRH-1 signaling node. This liver-centric pathway acts as a first-line checkpoint for intrahepatic and transhepatic BA flux upstream of the peripheral FXR-FGF15/19 pathway, which together constitutes an integral hepatoenteric control mechanism that fine-tunes BA homeostasis, counteracting cholestasis and hepatobiliary damage. Our findings shed light on potential therapeutic strategies for cholestatic diseases.
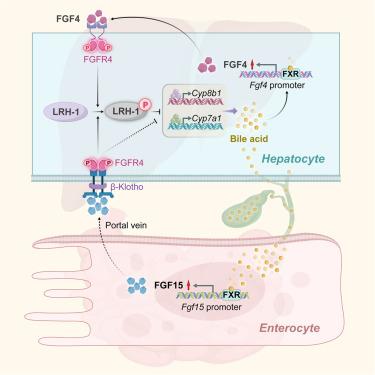
在胆汁淤积压力下,肝脏 FXR-FGF4 需要通过 FGFR4-LRH-1 信号节点实现胆汁酸平衡
胆汁酸(BA)稳态对各种生理过程至关重要,而胆汁酸紊乱则是胆汁淤积症的根源。法尼类固醇 X 受体(FXR)是通过回肠成纤维细胞生长因子(FGF)15/19 内分泌途径调节胆汁酸平衡的主调节器,对餐后或异常的经肠胆汁酸通量做出反应。然而,在非餐后或肝内胆汁淤积的情况下,肝脏 FXR 的从头旁分泌信号介质控制着肝脏内 BA 的合成程度,但这一介质仍然未知。我们发现肝脏 Fgf4 是 FXR 的直接靶点,它通过旁分泌信号下调 Cyp7a1 和 Cyp8b1。FXR-FGF4的作用是由未知的细胞内FGF受体4(FGFR4)-LRH-1信号节点介导的。这条以肝脏为中心的通路是肝内和跨肝 BA 通路的一线检查点,位于外周 FXR-FGF15/19 通路的上游,共同构成了一个完整的肝肠控制机制,可微调 BA 平衡,抵消胆汁淤积和肝胆损伤。我们的发现为胆汁淤积性疾病的潜在治疗策略提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


