Effect of mitochondrial translocator protein TSPO on LPS-induced cardiac dysfunction
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
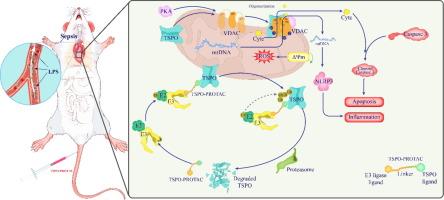
Sepsis-induced cardiac dysfunction is one of the most serious complications of sepsis. The mitochondrial translocator protein (TSPO), a mitochondrial outer membrane protein, is widely used as a diagnostic marker of inflammation-related diseases and can also lead to the release of inflammatory components. However, whether TSPO has a therapeutic effect on sepsis-induced cardiac dysfunction is unclear.Objectives
The aim of this study is to investigate the involvement of TSPO in the pathogenesis of sepsis-induced cardiac dysfunction and elucidate its underlying mechanism, as well as develop therapeutic strategies targeting TSPO for the prevention and treatment of sepsis-induced cardiac dysfunction.Methods
The sepsis-induced cardiac dysfunction model was established by intraperitoneal injection of lipopolysaccharide (LPS)in C57BL/6 mice (LPS-induced cardiac dysfunction, LICD). TSPO knockout mice were constructed,and the effects of TSPO was detected by survival rate, echocardiography, HE staining, mitochondrial electron microscopy, TUNEL staining. TSPO-binding proteins were identified by co-immunoprecipitation and mass spectrometry. The mechanisms underlying between TSPO and voltage-dependent anion channel (VDAC) was studied through western blot and immunofluorescence. Proteolysis-Targeting Chimeras (PROTAC) technology was used to construct TSPO-PROTAC molecules that can degrade TSPO.Results
Our present study found that LPS increased cardiac TSPO expression. Knockout of TSPO in C57BL/6 mice with LICD attenuated the cardiac pathology, mitochondrial dysfunction, and apoptosis of cardiomyocytes and significantly improved cardiac function and survival rate. Co-immunoprecipitation and mass spectrometry identified VDAC as a TSPO binding protein.Down-regulation of TSPO reduced PKA-mediated VDAC phosphorylation and VDAC oligomerization, ameliorated mitochondrial function, and reduced cardiomyocyte apoptosis. The study has clinical translational potential, because administration of TSPO-PROTAC to degrade TSPO improved cardiac function in mice with LICD.Conclusion
This study elucidated the effect of TSPO in LICD, providing a new therapeutic strategy to down-regulate TSPO by administration of TSPO-PROTAC for the prevention and treatment of LICD.
线粒体转运蛋白 TSPO 对 LPS 诱导的心脏功能障碍的影响
导言脓毒症引起的心脏功能障碍是脓毒症最严重的并发症之一。线粒体转运蛋白(TSPO)是一种线粒体外膜蛋白,被广泛用作炎症相关疾病的诊断标志物,也可导致炎症成分的释放。本研究旨在探讨 TSPO 参与脓毒症诱发心功能不全的发病机制,阐明其潜在机制,并开发针对 TSPO 的治疗策略,以预防和治疗脓毒症诱发的心功能不全。方法C57BL/6小鼠腹腔注射脂多糖(LPS)建立脓毒症诱导的心功能不全模型(LPS诱导的心功能不全,LICD)。通过存活率、超声心动图、HE 染色、线粒体电镜和 TUNEL 染色检测 TSPO 的影响。通过免疫共沉淀和质谱鉴定了TSPO结合蛋白。通过Western印迹和免疫荧光研究了TSPO与电压依赖性阴离子通道(VDAC)之间的内在机制。利用蛋白水解靶向嵌合体(PROTAC)技术构建了可降解 TSPO 的 TSPO-PROTAC 分子。结果本研究发现 LPS 增加了心脏 TSPO 的表达,用 LICD 在 C57BL/6 小鼠中敲除 TSPO 可减轻心脏病理变化、线粒体功能障碍和心肌细胞凋亡,并显著改善心脏功能和存活率。联合免疫沉淀和质谱分析发现 VDAC 是一种 TSPO 结合蛋白。下调 TSPO 可减少 PKA 介导的 VDAC 磷酸化和 VDAC 低聚化,改善线粒体功能,减少心肌细胞凋亡。该研究具有临床转化潜力,因为服用 TSPO-PROTAC 降解 TSPO 可改善 LICD 小鼠的心脏功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


