Activity-based protein profiling guided new target identification of quinazoline derivatives for expediting bactericide discovery: Activity-based protein profiling derived new target discovery of antibacterial quinazolines
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
The looming antibiotic-resistance problem has imposed an enormous crisis on global public health and agricultural development. Even worse, the evolution and widespread distribution of antibiotic-resistance elements in bacterial pathogens have made the resurgence of diseases that were once easily treatable deadly again. The development of antibiotics with novel mechanisms of action is urgently required.Objectives
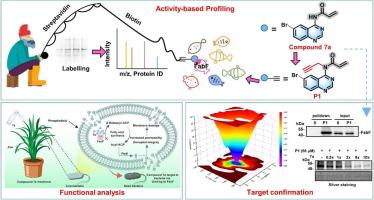
Inspired by charming activity-based protein profiling (ABPP) technology and increasing attention to quinazolines in the development of antibacterial agents, this study engineered a series of new quinazoline derivatives, assessed their antibacterial profiles, and first identified the possible target.Methods
The target identification and their possible binding sites were verified by ABPP technology, molecular docking, and molecular dynamic simulations. The fatty acid synthesis process was analyzed by gas chromatography, propidium iodide staining, and scanning electron microscopy. The physicochemical properties and fungicide-likeness were evaluated using the Fungicide Physicochemical-properties Analysis Database.Results
Compound 7a, an acrylamide-functionalized quinazoline derivative, exhibited excellent antibacterial potency against Xanthomonas oryzae pv. oryzae with an EC50 value of 13.20 µM. More importantly, ABPP technology showed that β-ketoacyl-ACP-synthase Ⅱ (FabF) was the first identified quinazolines’ potential target. Compound 7a could selectively bind to the Cys151 residue of FabF through covalent interaction, suppress fatty acid biosynthesis, and damage the cell membrane integrity, thereby killing the bacteria. The pot experiment results showed that compound 7a demonstrated protective and curative values of 49.55 % and 47.46 %, surpassing controls bismerthiazol and thiodiazole copper. Finally, compound 7a exhibited low toxicity towards non-target organisms. These unprecedented performances contributed to excavating new quinazoline-based bactericidal agents.Conclusion
Our research highlights the superiority of ABPP technology, for the first time, identifies the target of engineered quinazolines in pathogenic bacteria, and their potential target fished by ABPP tools holds great promise for the development of quinazoline-based and/or FabF-targeted bactericides.
基于蛋白质分析的喹唑啉衍生物新靶点鉴定加快了杀菌剂的发现:基于活性的蛋白质分析发现抗菌喹唑啉类化合物的新靶点
导言:迫在眉睫的抗生素耐药性问题给全球公共卫生和农业发展带来了巨大危机。更糟糕的是,细菌病原体中抗生素耐药性元素的进化和广泛分布,使得曾经容易治疗的疾病再次死灰复燃。本研究受迷人的基于活性的蛋白质分析(ABPP)技术的启发,以及喹唑啉类抗菌药在抗菌剂开发中日益受到重视,因此设计了一系列新的喹唑啉衍生物,评估了它们的抗菌谱,并首先确定了可能的靶点。方法通过 ABPP 技术、分子对接和分子动力学模拟验证了靶点的确定及其可能的结合位点。通过气相色谱法、碘化丙啶染色法和扫描电子显微镜分析了脂肪酸的合成过程。结果化合物 7a 是一种丙烯酰胺功能化的喹唑啉衍生物,对黄单胞菌(Xanthomonas oryzae pv. oryzae)表现出优异的抗菌效力,EC50 值为 13.20 µM。更重要的是,ABPP 技术表明,β-酮酰基-ACP-合成酶Ⅱ(FabF)是第一个确定的喹唑啉类药物潜在靶标。化合物 7a 能通过共价作用选择性地与 FabF 的 Cys151 残基结合,抑制脂肪酸的生物合成,破坏细胞膜的完整性,从而杀死细菌。盆栽实验结果表明,化合物 7a 的保护值和治疗值分别为 49.55 % 和 47.46 %,超过了对照组双苯噻唑和硫代噻唑铜。最后,化合物 7a 对非目标生物的毒性很低。我们的研究凸显了 ABPP 技术的优越性,首次确定了工程喹唑啉类药物在病原菌中的靶标,利用 ABPP 工具找到了它们的潜在靶标,为开发基于喹唑啉和/或 FabF 的靶向杀菌剂带来了巨大希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


