The nitration of SIRT6 aggravates neuronal damage during cerebral ischemia-reperfusion in rat
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ischemic stroke is a major cause of death and disability. The activation of neuronal nitric oxide synthase (nNOS) and the resulting production of nitric oxide (NO) via NMDA receptor-mediated calcium influx play an exacerbating role in cerebral ischemia reperfusion injury. The NO rapidly reacts with superoxide (O2−) to form peroxynitrite (ONOO−), a toxic molecule may modify proteins through tyrosine residue nitration, ultimately worsening neuronal damage. SIRT6 has been proven to be crucial in regulating cell proliferation, death, and aging in various pathological settings. We have previous reported that human SIRT6 tyrosine nitration decreased its intrinsic catalytic activity in vitro. However, the exact role of SIRT6 function in the process of cerebral ischemia reperfusion injury is not yet fully elucidated. Herein, we demonstrated that an increase in the nitration of SIRT6 led to reduce its enzymatic activity and aggravated hippocampal neuronal damage in a rat model of four-artery cerebral ischemia reperfusion. In addition, reducing SIRT6 nitration resulted in increase the activity of SIRT6, alleviating hippocampal neuronal damage. Moreover, SIRT6 nitration affected its downstream molecule activity such as PARP1 and GCN5, promoting the process of neuronal ischemic injury in rat hippocampus. Additionally, treatment with NMDA receptor antagonist MK801, or nNOS inhibitor 7-NI, and resveratrol (an antioxidant) diminished SIRT6 nitration and the catalytic activity of downstream molecules like PARP1 and GCN5, thereby reducing neuronal damage. Finally, in the biochemical regulation of SIRT6 activity, tyrosine 257 was essential for its activity and susceptibility to nitration. Replacing tyrosine 257 with phenylalanine in rat SIRT6 attenuated the death of SH-SY5Y neurocytes under oxygen-glucose deprivation (OGD) conditions. These results may offer further understanding of SIRT6 function in the pathogenesis of cerebral ischemic diseases.
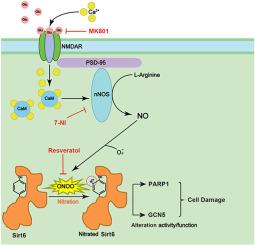
SIRT6 的硝化会加重大鼠脑缺血再灌注过程中神经元的损伤。
缺血性中风是导致死亡和残疾的主要原因。神经元一氧化氮合酶(nNOS)的激活以及由此产生的一氧化氮(NO)通过 NMDA 受体介导的钙离子流入在脑缺血再灌注损伤中起着加剧作用。一氧化氮会迅速与超氧化物(O2-)反应,形成过氧化亚硝酸盐(ONOO-),这种有毒分子会通过酪氨酸残基硝化作用改变蛋白质,最终加重神经元损伤。事实证明,SIRT6 在各种病理情况下调节细胞增殖、死亡和衰老至关重要。我们以前曾报道,人类 SIRT6 酪氨酸硝化会降低其体外固有催化活性。然而,SIRT6 功能在脑缺血再灌注损伤过程中的确切作用尚未完全阐明。在此,我们证明了在四动脉脑缺血再灌注大鼠模型中,SIRT6的硝化程度增加会导致其酶活性降低,并加重海马神经元损伤。此外,减少 SIRT6 硝化可提高 SIRT6 的活性,减轻海马神经元损伤。此外,SIRT6硝化还影响了其下游分子如PARP1和GCN5的活性,促进了大鼠海马神经元缺血损伤的进程。此外,使用 NMDA 受体拮抗剂 MK801 或 nNOS 抑制剂 7-NI 以及白藜芦醇(一种抗氧化剂)治疗可减少 SIRT6 硝化以及 PARP1 和 GCN5 等下游分子的催化活性,从而减轻神经元损伤。最后,在 SIRT6 活性的生化调控中,酪氨酸 257 对其活性和对硝化的敏感性至关重要。用苯丙氨酸替代大鼠 SIRT6 中的酪氨酸 257 可减轻 SH-SY5Y 神经细胞在氧-葡萄糖剥夺(OGD)条件下的死亡。这些结果可能有助于进一步了解 SIRT6 在脑缺血疾病发病机制中的功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nitric oxide : biology and chemistry
生物-生化与分子生物学
CiteScore
7.50
自引率
7.70%
发文量
74
审稿时长
52 days
期刊介绍:
Nitric Oxide includes original research, methodology papers and reviews relating to nitric oxide and other gasotransmitters such as hydrogen sulfide and carbon monoxide. Special emphasis is placed on the biological chemistry, physiology, pharmacology, enzymology and pathological significance of these molecules in human health and disease. The journal also accepts manuscripts relating to plant and microbial studies involving these molecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


