Establishment of a semi-continuous nano-production line using the Microfluidizer® technology for the fabrication of lipid-based nanoparticles part 1: Screening of critical parameters and design of experiment optimization studies
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
A variety of strategies for producing high-quality nanoparticles have been reported in recent years. Batch-based bottom-up and top-down technologies are generally the most efficient methods, but present a number of challenges, particularly in terms of variability, safety, sustainability and large-scale production. In this study, a scalable, semi-continuous production line was built by connecting individual processing units, including a high shear mixing device, the Microfluidizer® technology and a cooling system. Each unit was equipped with an adequate temperature control to allow solvent-free production of solid lipid nanoparticles (consisting of Precirol® ATO 5 or Gelucire® 43/01) and nanostructured lipid carriers (additionally comprising Labrafac™ lipophile WL 1349). Subsequently, critical formulation parameters and critical process parameters (CPPs) of the individual processing units and their effects on particle size (i.e., critical quality attribute (CQA)) were investigated to identify appropriate input parameters for the subsequent Design of Experiment (DoE) studies conducted after linking the process units to a semi-continuous production line. For particle size monitoring, spatially resolved dynamic light scattering (SR-DLS) measurements were conducted and compared to standard DLS measurements to evaluate the applicability of SR-DLS as an inline monitoring tool. It was found that matrix composition, emulsifier concentration, pressure and number of cycles when processing through Microfluidizer® processor were the most influencing parameters. By optimizing these parameters, five-times higher throughputs could be achieved by the semi-continuous manufacturing line. In addition, the particle size measurements with SR-DLS confirmed the feasibility of implementing this technology for real-time particle size monitoring as an important safety factor in quality control.
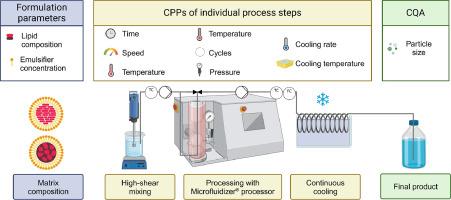
利用 Microfluidizer® 技术建立半连续纳米生产线,用于制造脂基纳米颗粒,第 1 部分:关键参数筛选和实验设计优化研究。
近年来,生产高质量纳米粒子的战略层出不穷。基于批量的自下而上和自上而下技术通常是最有效的方法,但也带来了许多挑战,特别是在可变性、安全性、可持续性和大规模生产方面。在这项研究中,通过连接各个处理单元,包括高剪切混合装置、微流控技术和冷却系统,建立了一条可扩展的半连续生产线。每个单元都配备了适当的温度控制装置,以实现无溶剂生产固体脂质纳米颗粒(包括 Precirol® ATO 5 或 Gelucire® 43/01)和纳米结构脂质载体(另外还包括 Labrafac™ 嗜脂剂 WL 1349)。随后,对各个加工单元的关键配方参数和关键工艺参数(CPPs)及其对粒度的影响(即关键质量属性(CQA))进行了研究,以便在将加工单元连接到半连续生产线后,为随后进行的实验设计(DoE)研究确定合适的输入参数。在粒度监测方面,进行了空间分辨动态光散射(SR-DLS)测量,并与标准 DLS 测量进行了比较,以评估 SR-DLS 作为在线监测工具的适用性。结果发现,通过 Microfluidizer® 处理器处理时,基质成分、乳化剂浓度、压力和循环次数是影响最大的参数。通过优化这些参数,半连续生产线的产量提高了五倍。此外,利用 SR-DLS 进行的粒度测量证实了采用这种技术进行实时粒度监测的可行性,因为它是质量控制中的一个重要安全因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


