Cell stress and phase separation stabilize the monomeric state of pseudoisocyanine chloride employed as a self-assembly crowding sensor
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cellular stress and ageing involve an increase in crowding and aggregation of amylogenic proteins. We here investigate if crowding is the intrinsic cause of aggregation and utilise a previously established non-protein aggregation sensor, namely pseudoisocyanine chloride (PIC). PIC shows fibrillization in cells into a highly fluorescent J-aggregated state and is sensitive to crowding. Surprisingly, cell stress conditions stabilise the monomeric rather than the aggregated state of PIC both in the cytoplasm and in stress granules. Regarding the different physiochemical changes of the cytoplasm occurring upon cell stress, involving volume reduction, phase separation and solidification, the intrinsic crowding effect is not the key factor to drive associated self-assembly processes. Cellular stress and ageing involve an increase in crowding and aggregation of amylogenic proteins, but the connection between protein destabilisation and the onset of aggregation is poorly understood. Here, the authors utilize a non-protein aggregation sensor based on pseudoisocyanine chloride to analyse the effect of macromolecular crowding in the cytoplasm on the self-assembly process, and find that the high crowding densities observed in the cytoplasm and stress granules upon stress are not an intrinsic cause for aggregation of amylogenic proteins.
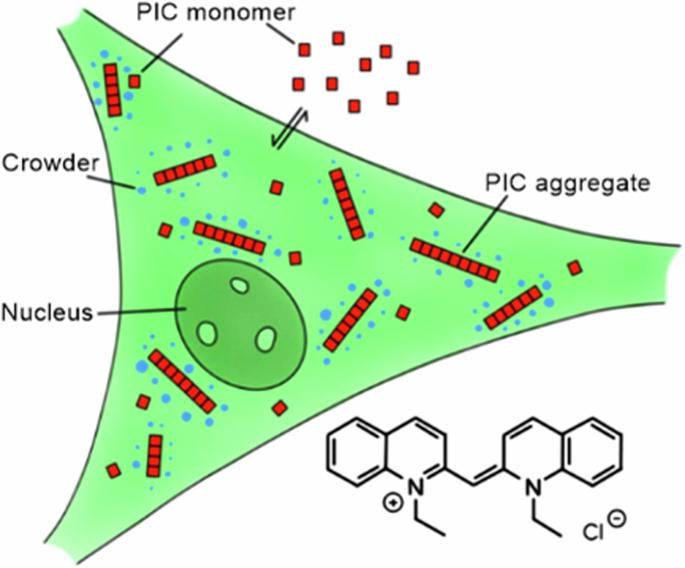
细胞应力和相分离稳定了用作自组装拥挤传感器的氯化假异堇的单体状态。
细胞应激和老化会导致淀粉样蛋白的拥挤和聚集。我们在此研究拥挤是否是导致聚集的内在原因,并利用以前建立的非蛋白质聚集传感器,即假异氰脲氯化物(PIC)。PIC 在细胞中显示出纤维化,形成高荧光的 J-聚集状态,并且对拥挤很敏感。令人惊讶的是,细胞应激条件会稳定 PIC 在细胞质和应激颗粒中的单体而非聚集状态。细胞应激时,细胞质会发生不同的理化变化,包括体积缩小、相分离和凝固,而内在的拥挤效应并不是驱动相关自组装过程的关键因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


