Regulatory role of Heparan sulfate in leptin signaling
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Leptin, a hormone mainly secreted by adipocytes, has attracted significant attention since its discovery in 1994. Initially known for its role in appetite suppression and energy regulation, leptin is now recognized for its influence on various physiological processes, including immune response, bone formation, and reproduction. It exerts its effects by binding to receptors and initiating an intracellular signaling cascade. Heparan sulfate (HS) is known to regulate the intracellular signaling of various ligands. HS is present as the glycan portion of HSPGs on cell surfaces and in intercellular spaces, with diverse structures due to extensive sulfation and epimerization. Although HS chains on HSPGs are involved in many physiological processes, the detailed effects of HS chains on leptin signaling are not well understood.
This study examined the role of HS chains on HSPGs in leptin signaling using Neuro2A cells expressing the full-length leptin receptor (LepR). We showed that cell surface HS was essential for efficient leptin signaling. Enzymatic degradation of HS significantly reduced leptin-induced phosphorylation of downstream molecules, such as signal transducer and activator of transcription 3 and p44/p42 Mitogen-activated protein kinase. In addition, HS regulated LepR expression and internalization, as treatment with HS-degrading enzymes decreased cell surface LepR. HS was also found to exhibit a weak interaction with LepR. Enzymatic removal of HS enhanced the interaction between LepR and low-density lipoprotein receptor-related protein 1, suggesting that HS negatively regulates this interaction. In conclusion, HS plays a significant role in modulating LepR availability on the cell surface, thereby influencing leptin signaling. These findings provide new insights into the complex regulation of leptin signaling and highlight potential therapeutic targets for metabolic disorders and obesity.
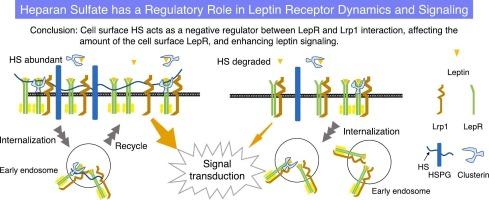
硫酸肝素在瘦素信号传导中的调节作用。
瘦素是一种主要由脂肪细胞分泌的激素,自 1994 年被发现以来一直备受关注。瘦素最初因其在抑制食欲和能量调节方面的作用而为人所知,现在人们认识到它对免疫反应、骨骼形成和生殖等各种生理过程都有影响。瘦素通过与受体结合并启动细胞内信号级联来发挥其作用。众所周知,硫酸肝素(HS)可调节各种配体的细胞内信号传导。HS 作为 HSPGs 的聚糖部分存在于细胞表面和细胞间隙中,由于广泛的硫酸化和外嵌合作用,其结构多种多样。虽然HSPGs上的HS链参与了许多生理过程,但HS链对瘦素信号转导的具体影响还不十分清楚。本研究利用表达全长瘦素受体(LepR)的 Neuro2A 细胞研究了 HSPG 上的 HS 链在瘦素信号转导中的作用。我们的研究表明,细胞表面的HS对有效的瘦素信号转导至关重要。酶降解HS可显著减少瘦素诱导的下游分子磷酸化,如信号转导和激活转录3以及p44/p42丝裂原活化蛋白激酶。此外,HS 还能调节 LepR 的表达和内化,因为用 HS 降解酶处理后,细胞表面的 LepR 会减少。研究还发现,HS与LepR之间存在微弱的相互作用。酶法去除 HS 可增强 LepR 与低密度脂蛋白受体相关蛋白 1 之间的相互作用,这表明 HS 负向调节了这种相互作用。总之,HS在调节细胞表面LepR的可用性,从而影响瘦素信号转导方面起着重要作用。这些发现为了解瘦素信号转导的复杂调控提供了新的视角,并突出了代谢紊乱和肥胖症的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


